Animalcules and Their Motors
In 1674, a Dutch cloth merchant in Delft, Antoni van Leeuwenhoek, spent his free time tinkering with lenses. One day, he pressed a drop of rainwater beneath his homemade microscope and observed what he called “animalcules” darting about. Leeuwenhoek described these creatures in two letters he sent to the Royal Society in London for publication, each replete with charming descriptions:
[T]he motion of most of these animalcules in the water was so swift, and so various upwards, downwards and round about that ‘twas wonderful to see: and I judged that some of these little creatures were above a thousand times smaller than the smallest ones I have ever yet seen upon the rind of cheese.
Leeuwenhoek may have been the first person to see microbes in motion, but his microscopes weren’t powerful enough to see the actual machinery responsible. (Leeuwenhoek mused that his animalcules might be using “little paws” to move.) It wasn’t until the 1830s that a German naturalist, Christian Gottfried Ehrenberg, saw whisker-like appendages, later named flagella, protruding from microbes. Still, the mechanism by which they worked remained unknown until the latter half of the 20th century, when electron microscopes finally homed in on the thousands of proteins that make a flagellar motor, able to convert flowing protons into mechanical motion.
%2C_Leyden%2C_1901-1930_Wellcome_L0057739.jpg)
Even more recently, a surge of research has revealed how evolution has finetuned the flagellum to operate in vastlydifferent ways based on a cell’s niche. Whereas an E. coli flagellum spins around nearly 20,000 times per minute, the flagellum in a microbe called Vibrio alginolyticus spins about five times faster, or slightly more than 100,000 times per minute. (For context, a Boeing 737 rotor has a maximum speed of 14,000 rpm.) This extra rotary speed is because Vibrio cells must traverse the ocean, where ion gradients (used to drive the motors) are large and nutrients more spread out.
A microbe found in the human digestive system, Campylobacter jejuni, also has a flagellar motor that generates much more torque than the one in E. coli — about 3,600 piconewton-nanometers. It uses this higher torque to propel itself through the viscous environment of the human gut.
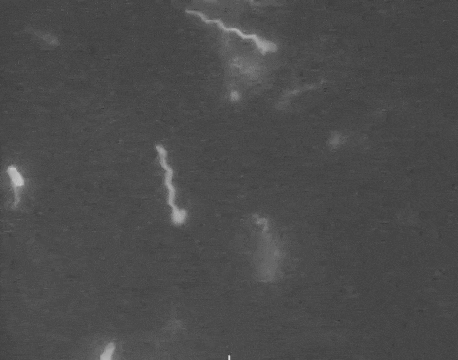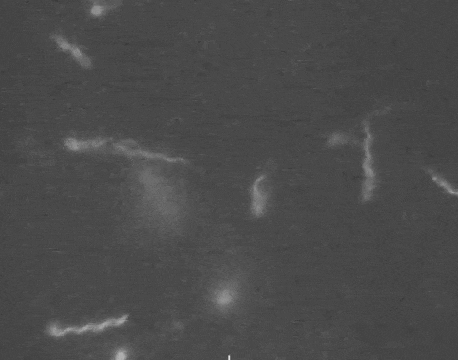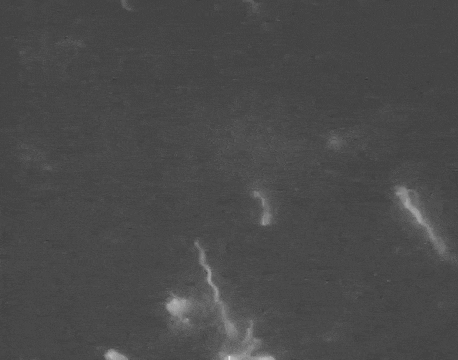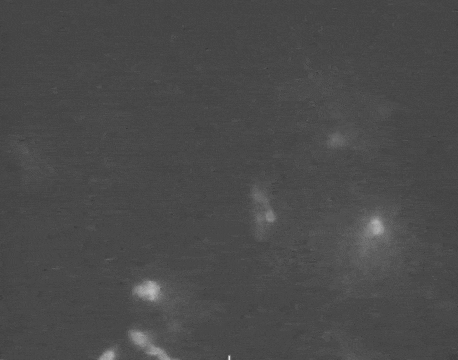
Earlier this year, a detailed structure of the high-torque C. jejuni motor was resolved for the first time. The structure revealed that the bacterium has extra motors located further away than normal from its central driveshaft. These motors push and spin the tail with greater leverage, and thus higher torque, than any other flagellum yet discovered.
This recent paper is just one contribution toward a structural-biology revolution that is finally shedding light on the molecular mechanisms underlying Leeuwenhoek’s 17th-century speculation about microbes’ “little paws.” It shows we’re still tackling a puzzle first uncovered more than 350 years ago.
{{signup}}
Cryo Structures
A flagellum looks deceptively simple from the outside (“just a long tail!”) but contains hundreds of interlocking proteins of many different types. Flagella have evolved independently at least three times — in bacteria, archaea, and eukaryotes — because they solve a fundamental problem for cells: namely, locomotion.1
Despite a high variance in structures, bacterial flagella are composed of four parts:
- the basal body, which makes up both the motor and driveshaft;
- stators, which convert the energy of flowing protons into torque;
- a flexible hook that connects the motor to the final part;
- a tail, which spins around like a corkscrew to push the cell forward.
The most amazing part of the flagellum is not only its composition of interlocking proteins or nature’s construction of literal motors, but rather that this mechanism self-assembles, piece by piece. Every protein in the flagellum fits perfectly to its neighbors. From within the cell, each protein finds its way to the growing structure, guided by transport systems that thread new parts through the base and out to the tip, where they click into place to complete the machine.
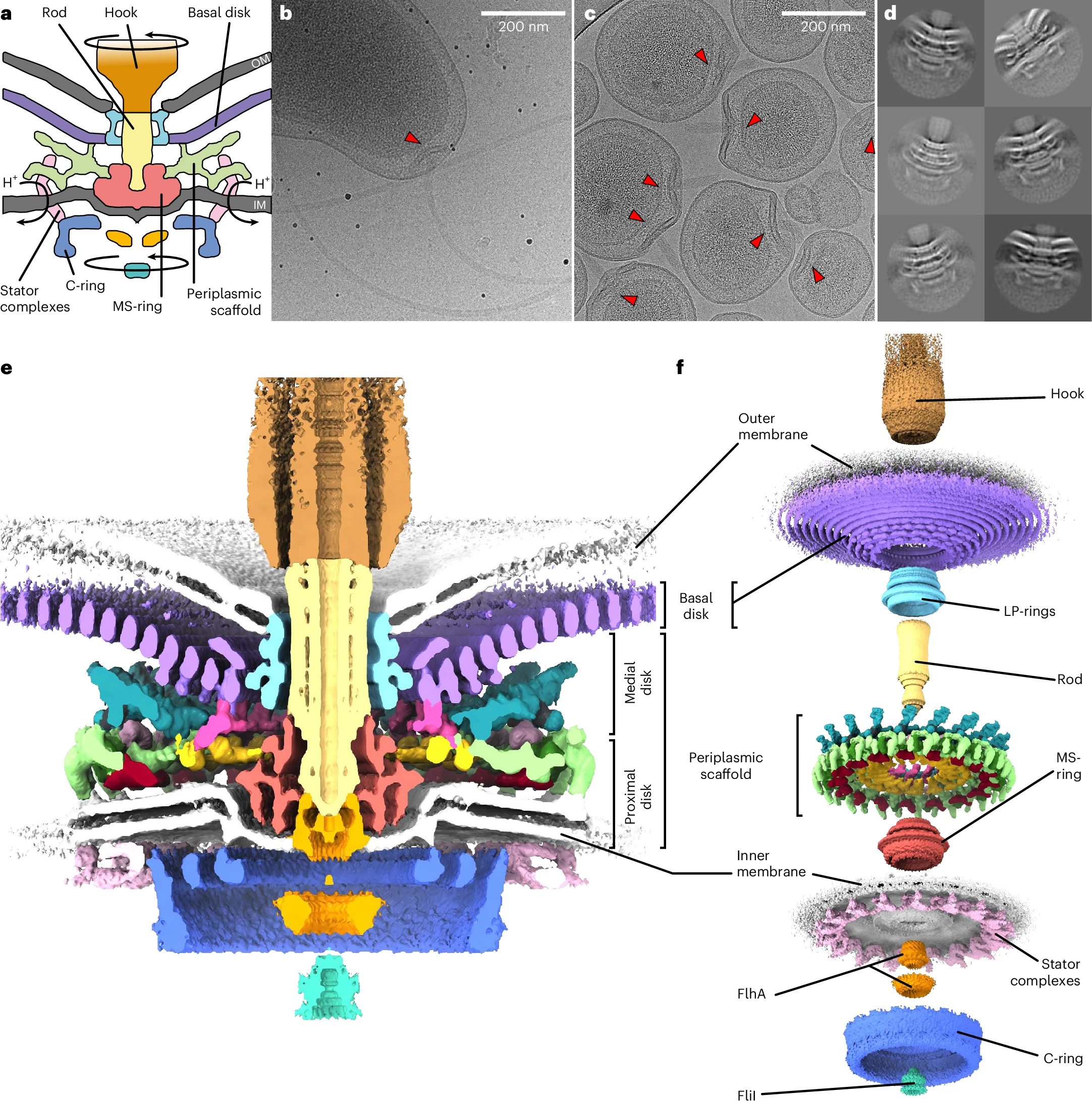
The working parts of the flagellum lie embedded inside the cell, spanning the inner membrane, periplasm (space between membranes), and outer membrane. When a bacterial flagellum assembles, it begins with the basal body, which is itself made from more than a hundred interlocking proteins and spans all three of these layers.
In 2021, Oxford researchers used cryo-electron microscopy to resolve the molecular details of the basal body from a microbe called Salmonella Typhimurium. The first part of the basal body, the C-ring, is located inside the cytoplasm just beneath the inner membrane and is built from three proteins — FliG, FliM, and FliN — repeated 34 times around the base. This structure converts torque from the stators into mechanical rotation on the rod.
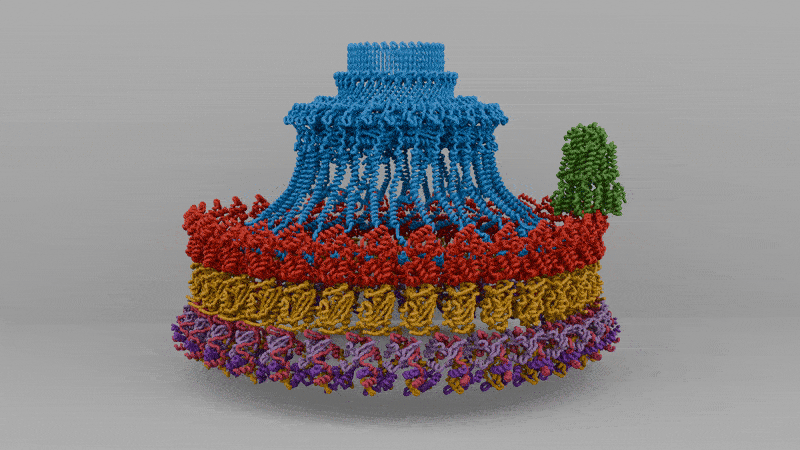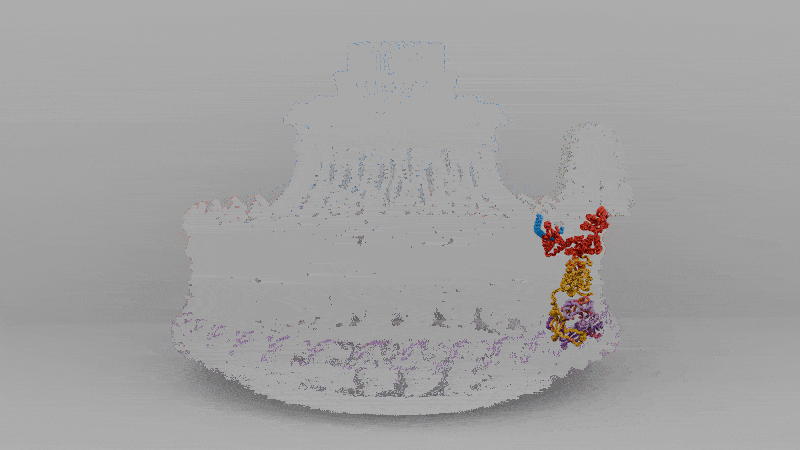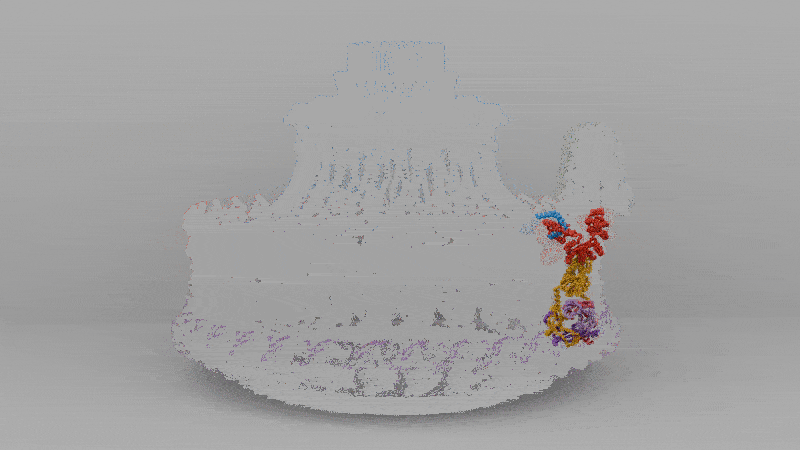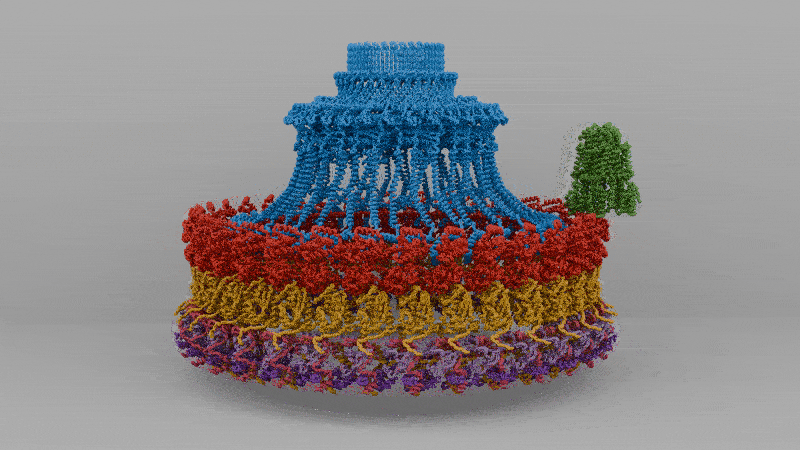
Above it lies the MS-ring, made entirely of a protein called FliF. About 34 FliF subunits assemble into a double-layered ring embedded in the inner membrane, acting together like a chassis to connect the C-ring in the cytoplasm to the drive shaft, or rod.
The rod itself is made from a rigid column of five proteins called FliE, FlgB, FlgC, FlgF, and FlgG. These proteins stack end to end, transmitting torque from the rotating MS-ring to the external hook and filament. Around the rod, two more rings, called the P-ring and L-ring, act as friction-reducing bearings, keeping the rod aligned as it spins thousands of times per minute.
The next piece of the flagellum is the hook, a short, curved segment that functions like a universal joint. It’s made from about 120 copies of a single protein, called FlgE, that hold the tail as it spins.
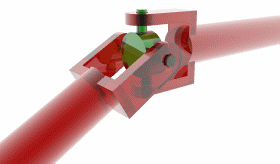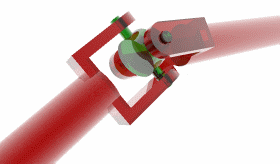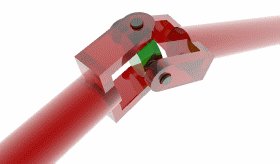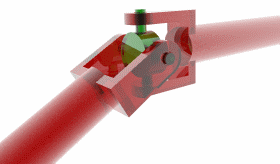
And then there is the tail itself, a helical propeller that spins like a corkscrew, pushing on the fluid in its viscous environment to drive its cell forward. A bacterial flagellum is typically up to ten micrometers in length, which, in E. coli, is significantly longer than the bacterium itself. Proportionally, this is akin to the tails of grass lizards or widowbirds, trailing three or four body lengths behind them.
Unlike animal tails, however, the flagellum is not a bundle of muscle or bone but rather a hollow cylinder of protein that builds itself, one subunit at a time. In fact, the tail is made almost entirely of repeating subunits of one protein, called flagellin. After the hook has self-assembled, the cell begins pumping unfolded flagellin proteins through it and into the hollow tail outside the cell. Each protein diffuses through this tail tube, reaches the growing tip, folds, and locks into place. As writer James Somers once quipped, “it’s as if the flagellum were built by vomiting forth parts of itself.”
Because the movement of each flagellin protein is diffusion-limited (meaning each protein drifts randomly through the narrow channel, rather than being actively pushed), each new protein needs to move further than the protein preceding it. And the further each flagellin protein must travel, the longer it takes the tail to grow. Longer flagella therefore elongate more slowly than shorter ones.

Finally, there are the stators, the actual motors that convert flowing protons into the mechanical energy that spins the tail.
It was long believed that the flagellum spins by burning ATP, an energy-storage molecule abundant in cells. But in 1977, researchers in Colorado did a clever experiment that disproved this. The researchers poisoned cells, collapsing the proton gradient without disturbing ATP levels. As soon as the poison was added, the cells stopped swimming. This showed that the flagellum is powered not by ATP, but rather by the flow of protons across the cell membrane.2
These protons flow from outside to inside a cell through small channels formed by two proteins embedded in the membrane, called MotA and MotB. Every time a proton passes through this channel, MotA changes its shape ever so slightly, nudging a protein embedded in the rotor, called FliG. This happens around a million times each second, collectively spinning the tail tens of thousands of times per minute.
These stators are incredibly energy efficient, too. It takes about 50 protons to power one revolution of a stator, with more than 90 percent of available energy being converted into mechanical work. To put that into context, a typical combustion engine converts only 20-30 percent of the chemical energy stored in gasoline into mechanical work at the crankshaft. The rest is lost to heat and friction. The cell as a whole swims far less efficiently, though. Only about 0.2 percent of energy is transferred into forward motion, because much is lost to the drag of water at microscopic scales.
But despite their elegant design, not all flagella are created equal. Different bacteria have adapted their flagellar motors for the worlds they inhabit, with some optimized for speed and others for force. One of the most striking variants, in my eyes, belongs to the microbe, Campylobacter jejuni, which uses its flagellum to drill through the viscous mucus of our intestines.
Evolving Torque
A C. jejuni cell looks a bit like a microscopic eel. Its name derives from the Greek kampylos and baktron, meaning “curved rod.” It is a pathogen, and one of the most common causes of food poisoning in the U.S. and Europe. This microbe thrives in the human gut; an environment far more viscous than water. To swim through it, C. jejuni has evolved a flagellum that has a torque far greater than the one in E. coli.
In July of this year, researchers at Imperial College London imaged the C. jejuni flagellum using cryo-electron microscopy, which works by rapidly freezing biological molecules into a thin layer of glass-like ice, trapping them in a nearly-native state. When an electron beam is shot through the frozen sample, particles strike the sample and scatter into a detector to form a noisy, two-dimensional image. By collecting hundreds or thousands of such images and aligning them computationally, researchers can calculate 3D maps of proteins.
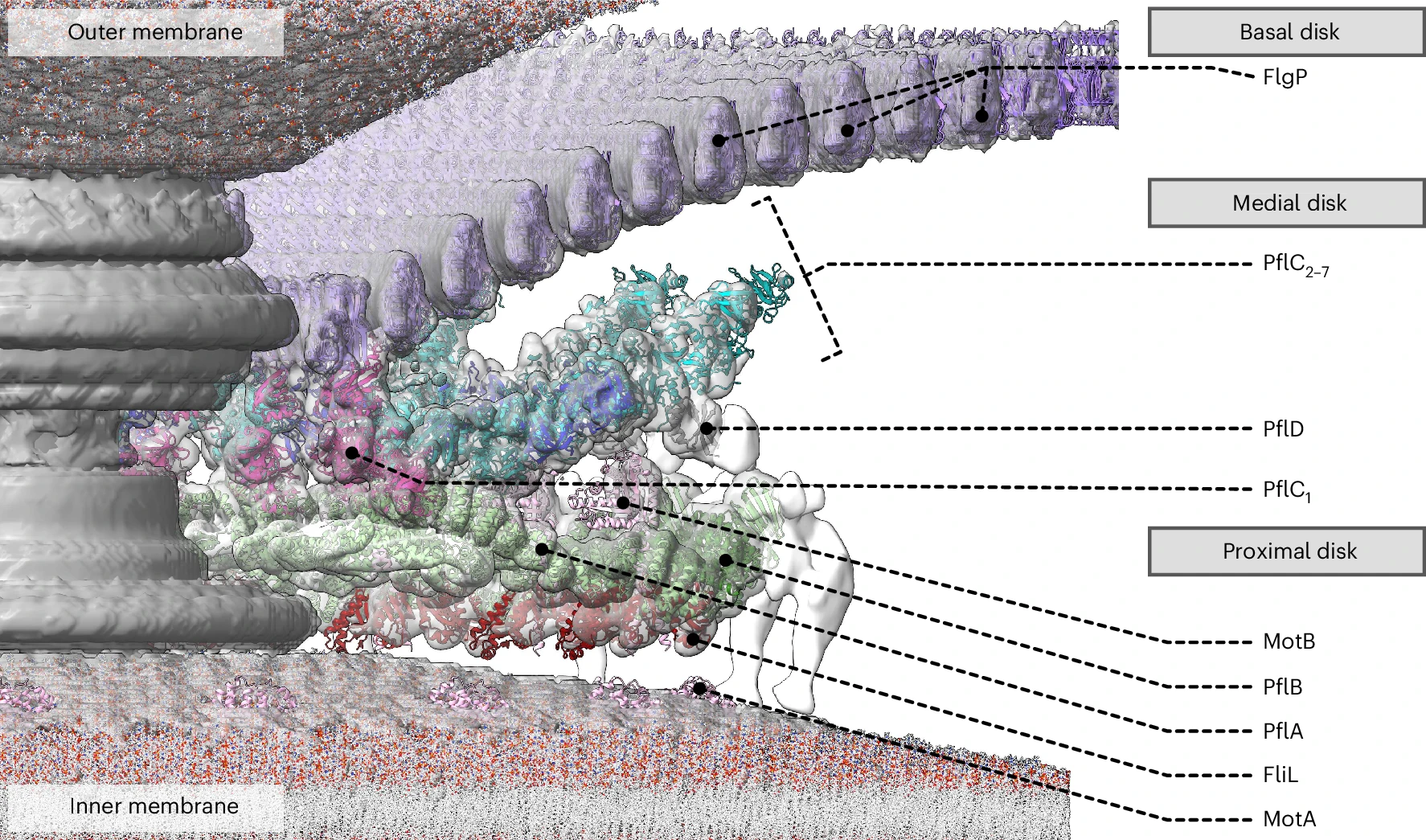
It wasn’t possible to solve the C. jejuni flagellum directly because it sits deeply embedded in the bacterial envelope, making the cells too thick for electrons to pass through. To surmount this problem, then, the researchers first coaxed the microbes to make “minicells,” basically tiny, round daughter cells carrying only a small amount of cytoplasm.
They did so by deleting a gene called flhG, which controls where the cell division machinery assembles, and other genes encoding flagellins, the proteins used to build the flagellum’s long tail. The result was a population of uniform, 200-nanometre-wide minicells, each carrying an intact, tailless flagellar motor near the surface. These minicells were thin enough for electrons to penetrate, thus allowing the researchers to image the motor at subnanometer resolution.3
The solved structure revealed that C. jejuni’s core motor isn’t that different, in principle, from E. coli’s, with a rotor in the middle and stators around it that act like little engines, each powered by flowing protons. But the C. jejuni flagellum has 17 stators compared to the 11 in E. coli. These stators are also positioned further away from the central driveshaft, or axle, by a large, newly-evolved scaffold structure, which enables them to exert more leverage.
Extra protein disks in their periplasm act as a cage to fix their stators in place, holding them at just the right distance from the rotor. In E. coli, the stators are loosely tethered and can drift in and out of position, so the motor runs below capacity. In C. jejuni, however, they’re locked down and permanently switched on. The end result of more engines, pushing from farther out, is three times the torque.
This paper on flagella is remarkable because it reveals how Darwin’s admiration of evolution’s “endless forms most beautiful” also applies at the molecular scale. The flagellum is not a single design but a spectrum of mechanical solutions to the same problem: how to best move a cell. What began in the 1600s as an investigation into the “little paws” that Van Leeuwenhoek could only imagine has evolved today into a high-resolution portrait of the proteins that drive their mechanisms.
{{signup}}
{{divider}}
Niko McCarty is a founding editor of Asimov Press.
Thanks to Morgan Beeby, associate professor of structural biology at Imperial College London, for helpful comments on this essay. Header image by Ella Watkins-Dulaney. Mistakes are my own.
Cite: McCarty, N. “Animalcules and Their Motors.” Asimov Press (2025). DOI: https://doi.org/10.62211/36qa-94vx
Footnotes
- Still, the basic principle — namely, a long tail that corkscrews around to drive cell movement — is found in everything from sperm cells to algae, mosses, and ferns. The Ginkgo biloba, a seeded plant, produces multiflagellate sperm, meaning each sperm cell carries tens of thousands of flagella arranged in spirals around its body. These flagella beat in coordinated waves to propel the sperm toward an egg. Ferns and mosses also have sperm cells with flagella. When water is present, the sperm use their flagella to swim from the male reproductive structure, over and across a thin film of water, into the female structure (the archegonium) where fertilization occurs.
- This is true in E. coli and S. Typhimurium, but some marine bacteria instead use sodium to power their flagella.
- Some of the protein structures were still blurry, so the researchers used AlphaFold to align the images and improve resolutions.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.