The Virus that Cures
It’s been over 25 years since the science magazine Discover first ran an extraordinary article about how a long-forgotten medical treatment, used in the former Soviet country of Georgia, could save us from the growing threat of untreatable, drug-resistant infections. In the country’s capital, Tbilisi, healthcare workers were using viruses that infect bacteria, known as bacteriophages (or just phages for short), to alleviate chronic infections.
With cases of drug-resistant infections on the rise, the article kick-started a renewed interest in this peculiar treatment, which had been virtually forgotten in the Western world since true chemical antibiotics, such as penicillin, became widely available in the 1940s. Soon after its publication, scientists, journalists, and investors were revisiting ‘phage therapy’ as a promising alternative to our failing antibiotics. A BBC documentary on ‘The Virus that Cures’ was broadcast around the world in 1997, and in the former communist party headquarters in Georgia, American investors helped organize the first major international conference on phage therapy since the 1930s. By 2004, over a dozen companies were working on developing phage products and therapies, in locations ranging from Baltimore to Bangalore, where ten years previously, there had been none.
Fast forward to 2023. At least a million people die from drug-resistant bacterial infections every year. Nearly three million drug-resistant infections occur annually in the U.S. alone, according to the Centers for Disease Control and Prevention. Many pathogenic bacteria have not just developed resistance to the most common drug used to treat them — known as antimicrobial resistance or AMR; they have become multi-drug resistant (MDR), extensively drug-resistant (XDR), or pan-drug resistant (PDR) — the latter meaning they are resistant to every drug we have in the cabinet.1
Yet even after more than two decades of research, media hype, and dozens of clinical trials and biotech start-ups that have come and gone, phage therapy has not scaled. No phage-based therapeutic has reached the latter stages of the clinical trial pipeline (where promising results lead to an expanded trial involving thousands of patients). Just a few of the 13 companies known to be working on phage therapy in 2004 are still active, most having pivoted to other uses of phages (for example, food decontamination) or disappeared. And despite numerous high-profile, individual success stories in U.S. and European clinics, the number of people able to access phage therapy outside the former Soviet Union is no more than a few hundred each year, rather than the many thousands or even millions who need it.
Answering the question of why phage therapy remains a niche treatment in the West requires that we first understand what makes phages so much more demanding to deploy than chemical antibiotics. It also asks us to work on ways to overcome these demands. Cutting-edge biotechnologies and emerging computing technology could finally transform this old therapeutic practice into 21st-century medicine. However, any strategy to scale phage therapy must still confront the fact that antimicrobial resistance is most keenly felt in low-income countries, and cutting-edge solutions are only effective if they are accessible and affordable to those who need them most.
Safety, Precision
The trouble with phage therapy is not that it doesn’t work. It’s been used both alongside and in place of antibiotics in parts of the former Soviet Union, including Poland, Czechoslovakia, Russia, and Georgia since the 1920s. There is also a growing body of successful treatments in the U.S., Europe, and China, where hundreds of individual cases suggest that phage therapies are safe and effective. Phage-based products have been licensed for use as antibacterial agents in the sanitation, food production, and animal health industries.
The problem is phages are nothing like the chemical antibiotics they are meant to replace. Once these remarkable-looking viruses attach to the outside of a host bacterial cell and inject them with their genes, they convert the host bacterium into a cellular factory for the creation of more viruses. These accumulated viruses destroy their hosts using a process called lysis, wherein lysin proteins rupture the cell membranes of their unfortunate hosts from within. A phage’s progeny then burst forth to seek out other bacterial cells to repeat the process. While antibiotics simply diffuse into bacterial cells, phages are living, evolving microbes that self-replicate wildly inside a patient’s body.
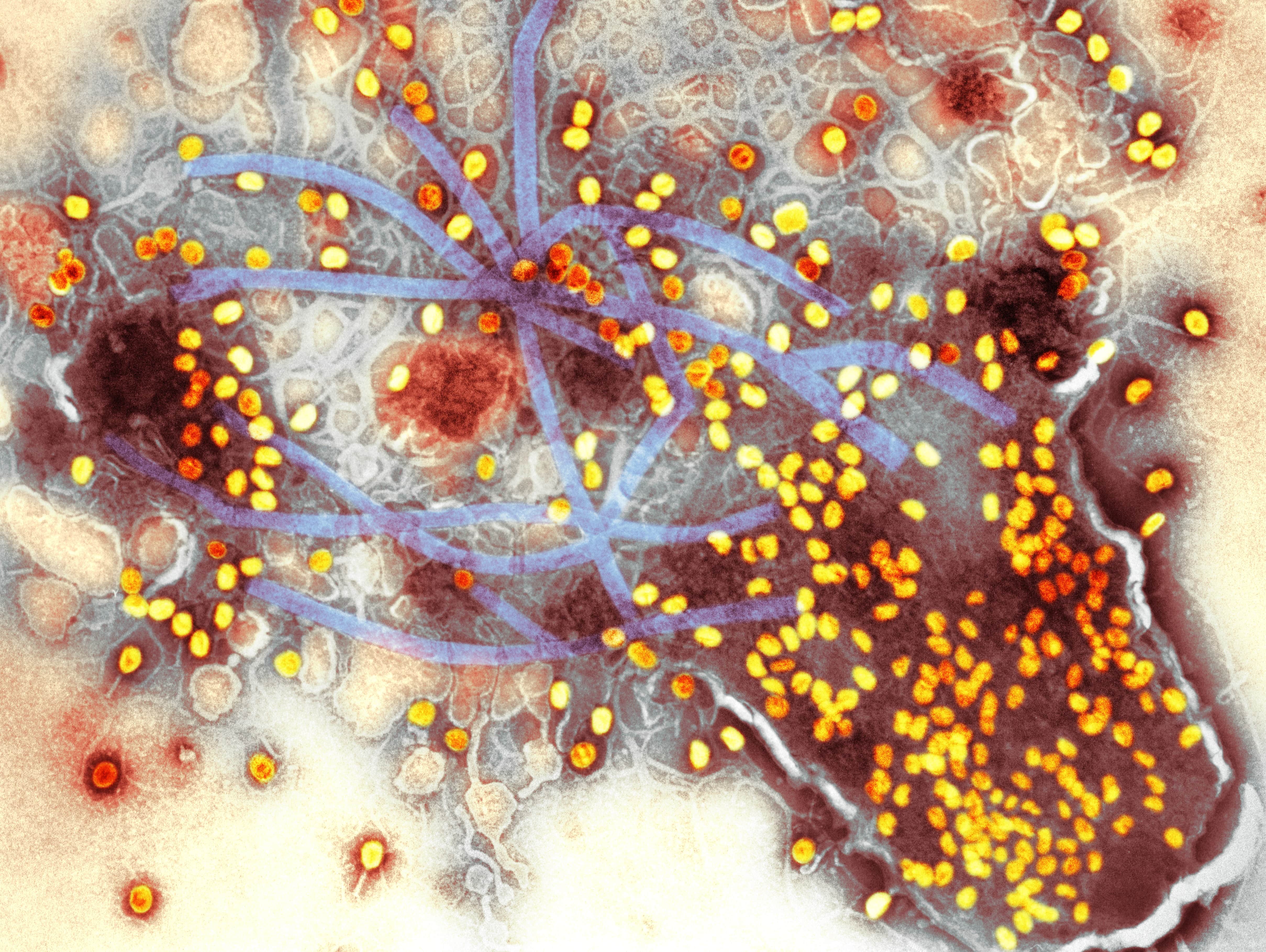
By far the biggest challenge with using phages as a drug is their specificity. There are estimated to be around 100 million different species of phage, and each one is incredibly choosy about which bacteria it will infect. Whereas an antibiotic will often work on dozens of different species of bacterial pathogen, each phage has evolved to target a single species of bacteria or, more often, specific strains of a single species.
Bacteria, having been beset by phages for billions of years, are wise to phage attacks. Cells can change the receptors that phages latch onto, either through mutations that alter a receptor’s shape or by using other proteins, embedded in the cell membrane, to mask those receptors. Sometimes bacteria will even jettison important elements of their cell wall, such as their protective outer capsule, or the fine hairs known as pili that help them move, if phages are binding onto them.
Over time, this dynamic leads to many, slightly different strains of both virus and host. Scientists often need to find several phages that can target a specific bacteria because cells can develop resistance to a single phage, or even to multiple phages in some cases.
Traditionally, scientists have worked through this problem in one of two ways. Either they engage in a cumbersome process to find phages that can infect and kill off the specific bacteria found in a patient — in essence, creating a bespoke treatment for every patient. Or, the scientists develop “cocktails” of several different phages (sometimes dozens) that, when mixed together, might work on a population of patients infected with different bacterial strains.
The former is labor-intensive, time-consuming, and therefore expensive. It’s virtually impossible to organize on a large scale. The phage cocktails, meanwhile, must be updated constantly to ensure they target the most common strains circulating in patients at any given time and place. This involves a certain amount of guesswork. Both often require clinicians to go out and find phages from the environment, or borrow them from collections in different countries, creating further bottlenecks as bacterial samples and phage strains are shipped back and forth around the world for tests.2
The specificity issue also makes it difficult to test phage therapies in a conventional clinical trial. Without strong clinical trial data, interest from investors is scant, limiting the scale and ambition of phage therapy research and the development of the purpose-built facilities needed to manufacture these complex medicines.
Although phages are generally safe — we live with billions of them inside and on us every day, after all – the large cohorts of patients that make up a clinical study mean they host many different strains of target bacteria, further complicating phage trials. Regulators traditionally want to see a single, stable, well-characterized drug before giving the green light for it to be tested in a clinical trial, not dozens of different viruses; let alone ones that are best found in unappealing places like sewage, hospital waste, or bird poop.
What’s more, making a concentrated solution of phages for medicinal use requires brewing them up in a big batch of the host bacteria, meaning every phage product is at risk of containing bacterial toxins and remnants of bacterial cells. Such contaminants could have the same toxic effects as the bacterial infection, or provoke a severe immune response in the patient, and therefore the phage mixtures must go through extensive purification and testing procedures. Phages are also prone to swapping genes with their bacterial hosts, so every phage that is used must be screened to ensure they are not carrying any genes that could inadvertently make the patient’s bacteria more dangerous.
Even in cases where researchers find phages that infect a particular type of bacterium, there are often subsequent steps required to make the phage sufficiently deadly to the cells. Phages are often ‘trained’ to inflict more damage to bacteria by repeatedly growing batches of the phage on the target bacteria and selecting the best performers generation after generation. But one can only train a phage so much before it has changed, genetically, to the point where it is now considered a different phage to the one the regulator initially approved.
Add these issues together, and the process of finding, manufacturing, testing and administering phages seems unfeasibly complex compared to a traditional antibiotic pill.
Still, researchers are trying. In one of the largest ever modern trials of phage therapy, a Є4m study organized by several national health agencies across Europe, phages were tested as a way to treat infected burns. It took two years to produce the phage cocktail to standards that regulators were happy with. Then, researchers were unable to find enough patients infected with the exact species of bacteria that the phage product was developed to fight. By the time enough patients were found and the manufacturing process was approved, the product being tested had lost titer – meaning the number of infectious phages in the mixture had fallen. The weakened dose of phages helped bacteria develop resistance, and the trial became yet another study that provided evidence phages are safe, but little more.3
Another considerable obstacle to pharmaceutical companies’ interest (and investment) is the fact that for a product to be patentable — and therefore profitable — it has to be considered new and inventive. A naturally occurring phage, or even a carefully curated mixture of them, is unlikely to be seen as either by patent offices.
Of course, these hurdles are not new. The pioneer of phage therapy, an eccentric self-taught scientist named Felix d’Herelle, understood even back in the 1920s that phage therapy was not a good fit for pharmaceutical developers seeking obvious routes to profit, or for healthcare systems looking for easy pharmaceutical fixes. These factors led d’Herelle to try and defect to the Soviet Union, which at the time was investing in enormous, nationalized healthcare infrastructure.4 But the same barriers still exist today.
Dr. Jean Paul Pirnay, a senior scientist based at Brussels’ Queen Astrid Military Hospital, has probably used phage therapy more than anyone else in the world outside of the former Soviet Union over the last decade. Thanks to his tireless efforts to advance phage therapy, and Belgium’s unusually progressive regulatory framework for this form of medicine, his team recently hit the milestone of 150 patients treated with phages.
Pirnay’s preliminary results are impressive; 77 of his first 100 patients reported clinical improvement, and complete eradication of the targeted bacteria was reported in 61 of them. And yet, even with Pirnay’s years of experience, his team’s streamlined manufacturing process, and Belgium’s supportive regulatory and quality control processes, it can still take weeks to make a phage preparation for each patient and get it approved by quality control agencies for use. (For life or death infections, such as sepsis, where a patient is likely to die imminently without treatment, scientists receive the necessary permissions in days, he says.)
Pirnay and his team are doing as much as they can to save lives on a case-by-case basis and build solid data on their work. But he is the first to admit that if phage therapy is ever to become a scaled-up, widely available form of medicine, the entire process needs a radical rethink.
Synthetic Phages
Artificial intelligence (AI) and machine learning are poised to solve at least one part of this multi-pronged puzzle: the need for scientists to test potentially hundreds of phages in a lab to find which ones will work on a given patient.
Various algorithms, trained on data from enormous databases of phages and bacteria, have been developed to predict phage-host relationships in silico rather than find them experimentally with lab work. Some algorithms simply look for important similarities between phage and bacterial DNA sequences, a signal of the extensive gene-swapping that goes on when a particular virus and bacteria have shared history. Others take the DNA sequences of known phage-host pairs, or the protein sequences of compatible binding sites and receptors, and then use deep-learning to make predictions about other phages and their hosts. Integrating these different approaches can predict phage-host matches with ‘false discovery’ rates of less than 10 percent.
With an eye on tools more applicable to a clinical setting, some algorithms are trained on one particular group of pathogenic bacteria. Last year researchers from Ghent University and the University of Valencia published details of a tool trained just on data from phages that can infect Klebsiella bacteria, a microbe that can cause pneumonia or enter wounds and cause sepsis, or blood infections. Klebsiella pneumoniae is one of the most prominent multidrug-resistant pathogens worldwide, and the researchers say their model can predict which phages will infect specific clinical strains of Klebsiella pneumoniae with an accuracy of 83 percent, a number that has been validated by testing the predicted phages on actual patient samples in the lab.
If and when predictive tools are used to find phages for patients in a real clinical case, the ‘predicted phages’ will still need to be tested on each patient’s bacterial sample first, but the discovery stage of the therapy could be cut down to minutes, rather than weeks or months of lab work.
But Pirnay hopes to go a step further. He believes the only way phage therapy can realistically be delivered at scale is by using AI and synthetic phages, manufactured close to where they will be administered to treat a patient.
“As you know, the big problem is, we're limited in what we can do [with phages] by the number of people that can make these products, and then you have the shipment of bacterial strains, shipments of phages between countries with different regulators,” says Pirnay. Phages that can be essentially downloaded and synthesized by anyone, in any location, would solve those problems, he says.
This process would begin by taking a sample of bacteria from the patient and sequencing its DNA. A computer algorithm would take that sequence and compute the DNA sequence of a phage best suited to killing it. This ‘ideal phage’ would then be manufactured from scratch, using synthesized DNA.
The tech for this process already exists. Extracting and sequencing DNA from biological samples is now so commonplace that it can be done in the middle of the jungle with a portable kit that fits in a backpack; the algorithms to predict phage-host relationships are becoming ever-more accurate, as described earlier, and the first phage made with DNA synthesized chemically in a lab was made over twenty years ago.
While phages and other viruses have been made from synthetic DNA for a long while, most of these viruses were still produced by a bacteria ‘infected’ with the synthetic DNA, a process which requires maturation in the cell. But Pirnay and colleagues are now able to produce entirely synthetic phages using what is known as cell-free bacteriophage synthesis.
This process, developed by researchers at the Technical University of Munich, involves chemically synthesizing the phage DNA and then, in a series of complex chemical steps, using that sequence to produce and assemble the protein parts of the viruses, replicating what would happen inside an infected bacterial cell. Pirnay describes it as being like a ‘printer, or espresso maker’ for viruses.
Pirnay and his colleagues across Europe hope to perform the first therapy with a phage synthesized using this method in the next few months.
The promise of synthetic phages is clear: beyond being manufactured when and where they are needed from just a digital DNA sequence, synthetic phages could be customized to fit the exact criteria demanded by a patient. In contrast to naturally occurring phages, which often contain extra genes that we know little about, synthetic viruses could be extremely minimalist, with only the genes strictly necessary to infect and kill the bacteria at hand. Knowing exactly what genes are in each phage, and exactly what they do, could make them more predictable and safer to use. Crucially, it could also make them easier to license as therapeutic agents. Manufacturing phages without bacterial host cells would also reduce concerns over the final product being contaminated with bacterial toxins.
Only synthetic phages, Pirnay says, can remove the logistical hurdles of finding phages in banks, testing them in labs, and shipping them around the world. To the question of how to turn this from an academic project into a marketable product, he suggests that a complete phage-testing and synthesis system, or a subscription-like service – with access to the phage-matching algorithms and all the necessary genetic data – could eventually attract major investors.
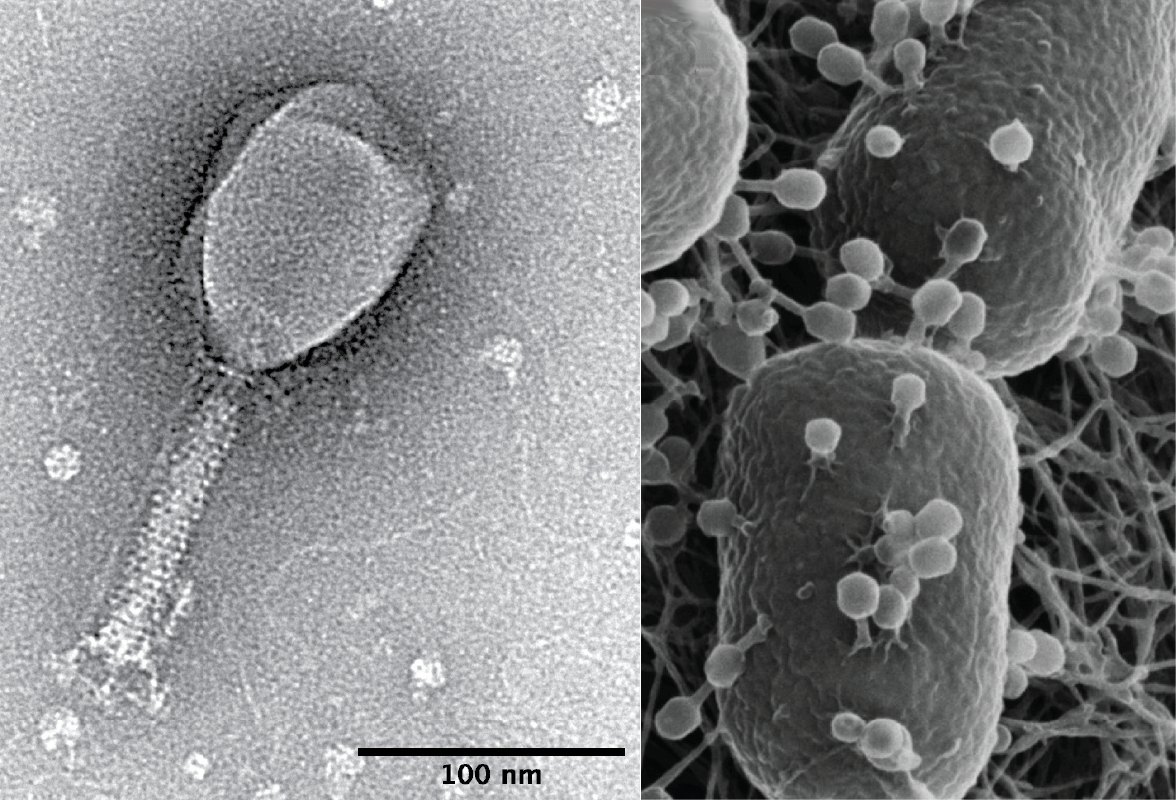
Off-the-Shelf Therapies
Others remain convinced that it is possible to channel the bacteria-lysing power of phages into more conventional ‘off-the-shelf’ therapeutics. Of the many phage companies to have emerged over the last 20 years, one of the few that has attracted serious backing from investors is Locus Biosciences. With $80m of funding from Johnson and Johnson, Locus has developed and merged high-tech platforms involving robots, CRISPR gene-editing, and AI, which the company says can help them solve the specificity issue, creating a phage product that works on virtually all strains of a given bacteria.
The Locus Platform uses automation and machine learning to create and test phage combinations, which according to their CEO Paul Garofolo, can infect and kill up to 95 percent of the microbial strains they are targeting, in contrast to bespoke therapies that target single strains, or products that have failed to work convincingly in clinical trials.
Locus has products in development targeting four common sources of bacterial infections: E. coli, P. aeruginosa, S. aureus, and K. pneumoniae. Their platform allows them to test thousands of phages at a time on hundreds of different bacterial strains. The resulting data are fed into an analytical engine that runs millions of simulations.
The result predicts a combination of phages that would ensure the broadest possible coverage of different bacterial strains. However, this isn’t just about sheer numbers – a mixture with hundreds of different phages would be far too complex for the company to manufacture, and so their algorithms aim to get the number of phages in their cocktails as low as possible, typically resulting in mixtures of around six.
Getting infectivity across all possible strains is step one – you also need to ensure the phages actually kill all the problematic bacteria, too. Using CRISPR-Cas3, one of the family of CRISPR enzymes that allow scientists to make precise genetic changes to DNA, Locus can add genes to their phages that not only help to modify their host range but confer additional bacteria-killing power. This might be the addition of genes that cause infected bacteria to secrete toxic compounds, genes that help the phages survive longer in the bloodstream or genes that boost the phages’ ability to fight through ‘biofilms’ – the tough slimy matrix that grows around and protects colonies of bacterial cells.
In a trial of their most advanced asset, a phage mixture targeting E. coli in urinary tract infections, Garofolo says they achieved a 6-log reduction in bacterial numbers in patients (meaning the number of bacterial cells present has been reduced a million-fold), leading to “100 percent clinical cure” of all 31 patients. Phase II clinical trials involving almost 300 patients are due to begin in April.
Garofolo is brutally frank about the failures of phage therapy research to translate into viable therapeutic products in decades past. He says that too much of the patchy money coming into phage therapy, often from charities and public research grants, has been spent on organizing clinical trials that are beset by the same familiar obstacles, instead of being directed toward technologies that can overcome them.
“There is a trend across the industry where an academic takes a few phages that work nicely on a few strains in the lab and decide that they have a company,” says Garofolo. “They spend all the money they get on organizing a trial, and that’s the kind of data that’s been coming out. And it sucks!”
“Take E. coli, for example, where you have 187 serotypes, or potential substrains, within that species. You have to be able to hit at least 95 percent of those strains to have anything that is comparable to an antibiotic. You need millions of dollars of investment to build diverse panels of temporally current phage strains, not a few 15-year-old, beat-down strains from an academic lab.”
A range of other start-ups have recently emerged taking the high-tech approach: Felix Biotech, based in San Francisco, is using advanced genetic engineering and cell-free manufacturing to create engineered phages with vastly broader host ranges than can be found in nature, and which also carry bacteria-killing compounds. Tolka AI, meanwhile – formed by two former Google engineers who have suffered chronic, drug-resistant infections – uses machine learning and the latest in biotech automation to take the labor out of creating bespoke phage therapies for individual patients. (Its CEO, Johan Wilkstrom, is due to be the company’s first patient after the FDA recently granted them a license for compassionate use, known as an expanded access application.)

Garafolo believes that if any of these new approaches to phage therapy can lead to just one impressive result in a major trial, it could open the floodgates to robust investment in phage therapy from the world’s biggest drug makers. “You know, any time there is a completely new modality, nobody really pays attention except for a couple of believers, until you get that first win, and then it really opens up. What do you think people said about CAR T-cell therapy when it was first suggested that they wanted to drain all the blood out of you like a vampire, treat it outside your body and then put it back in?”
“Somebody’s just got to break through with good data, even if it’s not us. Because frankly, do you know of anything else that can work against multiple drug-resistant bacteria? No.”
A Global View
Even as advances in computing and molecular biology usher in the era of a new, high-tech “phage therapy 2.0”, it is worth noting that the burden of antimicrobial resistance (AMR) is predicted to fall most heavily on low-income countries. An oft-cited estimate is that by 2050, at least ten million people could die each year from antibiotic-resistant infections. But what is less well-publicized is that the same 2016 review predicted that the vast majority of these deaths – up to 90 percent – will occur in Africa and Asia, where antibiotic use is soaring and access to alternatives is poor.
Therefore, while the development of high-tech phage platforms in the U.S., Europe, and China is encouraging, these new approaches might not necessarily translate into affordable products suitable for a global health crisis. One start-up operating in the U.S. told me that their high-efficiency robotics-led platform, which creates bespoke phage products based on automated analysis of patients’ bacterial samples, is likely to work out in the region of $20,000 per treatment. That may be cost-effective for U.S. insurers wanting to resolve long-term infections, especially those that would otherwise lead to amputations. But it remains vastly more expensive than a generic antibiotic, which might cost as little as a few cents per dose, meaning it is likely to remain a last resort treatment, even in the relatively affluent U.S. Administering phage therapy also requires cold storage and transport, constant pathogen surveillance and testing, and purpose-built manufacturing facilities, all more costly than the current, if increasingly ineffectual, alternative.
Phage therapies matched to individual patients and requiring bleeding edge technologies may never be economically or logistically viable in low and middle-income countries. But thankfully there are groups — from charities and start-ups to the non-profit arms of major pharma companies — looking specifically at more public-health-focused approaches to scaling phage therapy for these parts of the world.
One method is to use phages as preventative or prophylactic treatments that can be distributed to slow or stop the spread of bacterial disease. Diseases like TB and cholera, hugely prevalent in Africa and Asia, are particularly compatible with these approaches: anti-TB phages tend to have broader host ranges than phages of other bacterial species, and strains of cholera bacteria are genetically very similar across the globe, increasing the chances that a cocktail of phages could be developed for these diseases that work across many patients and potentially even across entire continents.
The start-up PhagePro Inc has a prophylactic phage cocktail for the preventative treatment of cholera that is currently in preclinical development; the cocktail is not only geared to target Vibrio cholera in certain vulnerable locations but has been formulated into dehydrated doses that allow for storage and distribution without refrigeration. The charity Phages for Global Health is also working on preventative cholera treatments that could be used to decontaminate water supplies and is using phages to decontaminate poultry farms of Campylobacter bacteria, the leading cause of diarrhoeal disease in Africa.
Alongside the development of phage products, Phages for Global Health is helping to develop an ‘ecosystem’ of skilled phage hunters, phage biobanks, and manufacturing facilities across these vast regions, ready to be put into action as the looming crisis of drug resistance deepens. Although developing phages into a clinically proven pharmaceutical product is dizzyingly complex, finding and culturing clinically relevant phages from the local environment is comparatively inexpensive — it is essentially the same microbial methodology used in the 1920s.
Important, pathogen-destroying phages have even been found by students and members of the public as part of citizen science projects.5 One simply takes a sample of dirty water or soil, filters out anything bigger than a virus, and then washes the resulting solution over a plate of bacteria. If tiny holes start to appear (signaling microscopic viral epidemics spreading among the bacterial cells), you have a phage that infects that bacteria. Find lots of phages that each work on different strains of bacteria, throw them in together, and you have a more low-tech, Soviet-style cocktail — which may not survive the rigors of a Western clinical trial but has worked well enough for clinicians elsewhere to use for over a century.
In places like Tbilisi, Georgia, these relatively low-tech phage cocktails might contain upwards of 30 different phages, targeting the most common bacterial causes of very broad conditions (i.e. they have one product for intestinal infections, one for skin infections, and so on.) The phages in these mixtures are not well-studied or understood before being added to the mixtures, and their efficacy is rarely tested, which is one of the main reasons Western scientists and drug regulators don’t advocate their use. But just slightly more scientifically rigorous adjustments to this old Soviet method could be enough to create safe, affordable, and locally relevant products in low and middle-income countries, says Tobi Nagel, founder of Phages for Global Health.
“If the Georgians [genetically] sequenced their phages, screened them for dangerous genes, that would go a long way towards the quality control that's needed,” she says.
Meanwhile, national and international pathogen surveillance systems, set up to monitor the spread of viruses like HIV, and more recently AMR, could be easily adapted to test locally sourced phages against locally circulating strains of drug-resistant pathogens. If phage medicines for priority diseases can be licensed through initiatives like the World Health Organisation Pre Qualification, it could negate the need for regulatory approval in individual countries.
Of course, the models for low and middle-income countries will be heavily reliant on public or philanthropic funding, rather than the profit incentive that drives so much drug development in the West. ‘Big funders’ are starting to direct more and more public-health funding towards phage therapy projects in low and middle-income countries, says Nagel, but it will need to increase vastly, and quickly, if projections about AMR deaths in Africa and Asia prove accurate.
No Magic Bullets
Despite the exciting progress towards scaling phage therapy described in this piece, even the most high-tech phage therapy products in development won’t deliver a ‘magic bullet’ against bacterial disease like the first chemical antibiotics did in the 1940s and 1950s. For a start, all of the current products approaching or in clinical trials target just eight highly prevalent bacterial species that cause infections, for a handful of specific indications (i.e. UTIs, diabetic foot ulcers). Doctors commonly encounter at least 13 or 14 bacterial species with drug-resistant strains across a range of infections in different parts of the body. Some of these infections are hugely complex: the mucus-filled lungs of cystic fibrosis patients, for example, can often be colonized by several species of drug-resistant bacteria, with multiple strains of each species, all long established and deeply embedded into the tissue and surrounded by a thick, sticky biofilm.
It’s likely that different approaches will be required for different infections. Broad-acting, marketable cocktails may become available for common infections, but more personalized or bespoke phage treatments using engineered or synthetic phages will be needed for acute, rare, or complex conditions.
Phages will also likely be used in combination with existing antibiotics, rather than replace them. Research suggests that combination therapy, using phages and antibiotics together, causes drug-resistant bacteria to focus so much on repelling the phages that they become resensitized to the antibiotics. (I’m told phage researcher Dr. Sabrina Green wants to appropriate the phrase MAGA for phages – because they can “make antibiotics great again”.)
In one interesting example from Belgium, researchers found that the key mutation that was allowing bacteria to evade phage infection was the loss of an efflux pump, a kind of protein that the bacterium uses to bail antibiotic compounds out of the cell and into the environment. In becoming resistant to the phage, the bacterium is unable to pump out antibiotics.
In another example, the notoriously hard-to-treat bacteria, Acetinobacter baumannii, evolved to escape phage attacks by ditching its entire protective outer capsule, making it highly susceptible to common beta-lactam antibiotics. The more that phages and phage therapy can be understood in this degree of molecular detail, the more data will be available to develop synergistic combination therapies that reduce the possibility that bacteria can evade both.6
But excitingly, if phages do become recognized as a form of therapeutic, then their use is not just limited to treating bacterial infections. Phages are, essentially, relatively non-toxic, nano-sized syringes, highly evolved to find a very particular target and deliver a payload. That means they could be used to deliver anything from anticancer compounds to mutation-correcting gene therapies, injecting the goods directly into the cells that need them. Already, groups are working on phage-based drug delivery vehicles and even phages that can form scaffolds in the body to repair tissue, for example after strokes. Precision treatment of the gut microbiome is also an exciting future application; phages can be used to wipe out problematic bacterial species while probiotics are used to add back good ones — although the science of the gut virome remains in its infancy.
However, before that can happen there is the immediate challenge of being able to test phage products. As I finalized this piece, a pioneer in the development of phage therapy messaged me to say that one of his patients had just died from a complex and multi-species bacterial infection. Despite his team having identified over a hundred potential phages to treat the infection, there simply wasn’t a regulatory framework available to permit their use. This is what stands in the way of scaling phage therapy.
AI and synthetic biology undoubtedly offer the best hope of engineering phages into products with the broad and versatile power of antibiotics. But for phage therapy to scale, we need a global coalition of scientists, drug developers, public health experts, and both public and private funding to come together to solve the unique challenges of licensing and regulation in different parts of the world. We will also need phage therapeutics that do not cost tens of thousands of dollars per treatment and are accessible to those in regions bearing the brunt of AMR. Otherwise, the sad story above will be repeated many, many times over.
But even for all the interconnected challenges, it’s worth reminding ourselves that every day, we are surrounded by hyper-diverse, hyper-abundant, and readily programmable bacteria killers. Phages have evolved time and time again to overcome bacteria’s wily knack for developing resistance, offering hope that we can, too.
***
Tom Ireland is an award-winning science journalist. He is editor of The Biologist, a magazine published by the Royal Society of Biology, and author of The Good Virus: The Amazing Story and Forgotten Promise of the Phage.
Cite this essay: Tom Ireland. "Scaling Phage Therapy." Asimov Press (2024). DOI: https://doi.org/10.62211/82rs-14tp
Header image: David S. Goodsell, RCSB Protein Data Bank and Scripps Research. doi: 10.2210/rcsb_pdb/goodsell-gallery-048
***
Footnotes
- Bacteria can acquire resistance to antibiotics in many different ways: some chance upon a helpful genetic mutation that makes them less susceptible to the antibiotic, some have dormant genes that help them resist toxic compounds that they can reactivate in the presence of an antibiotic, and many bacteria can trade useful genes with nearby bacterial cells in a process called horizontal gene transfer, meaning resistance genes can spread among unrelated bacterial cells of different species. Resistance can take many forms, from the production of metabolites that neutralize or modify the antibiotic compounds, to protein pumps that help physically to bail the antibiotic out of the cell. To learn more about how bacteria and other microbes develop resistance, check out the CDC’s information sheet on AMR.
- As you might imagine, getting packages marked ‘viruses’ through customs is a problem in many countries, requiring special permissions or fees. I cover this more extensively in The Good Virus.
- This review of phage therapy trials from 2021 concluded that over a dozen phage therapy trials have proven the treatment is safe, but none have conclusively demonstrated efficacy.
- d’Herelle’s plan to move to Georgia was thwarted when Stalin had his Georgian scientific partner executed. He prudently decided to stay in France.
- A key phage known as ‘muddy’ used in several successful phage therapy cases in the US, was found on a rotting aubergine by a South African student. And by taking water samples from a tiny stream near my house, I helped scientists from the Citizen Phage Library Project find a new species of phage that kills E.coli, was effective against clinical samples, and has been banked for future clinical use.
- Interestingly, some of the most exciting new antibiotic drugs in development are based on the lysin enzymes that phages use to burst their hosts open.
Always free. No ads. Richly storied.