The Penicillin Myth

<iframe data-testid="embed-iframe" style="border-radius:12px" src="https://open.spotify.com/embed/episode/1sI1gh2anhxjYRmTXJ4Ch4?utm_source=generator" width="100%" height="152" frameBorder="0" allowfullscreen="" allow="autoplay; clipboard-write; encrypted-media; fullscreen; picture-in-picture" loading="lazy"></iframe>
“I did not invent penicillin. Nature did that. I only discovered it by accident.”
—Alexander Fleming
Many know the story of Alexander Fleming’s chance discovery of penicillin. Fleming, a bit of an absent-minded professor (and a bit of a slob), left culture plates streaked with Staphylococcus on his lab bench while he went away on summer holiday. When he returned, he found that “a mould” had contaminated one of his plates, probably having floated in from an open window. Before discarding the plate, he noticed that, within a “ring of death” around the mold, the bacteria had disappeared. Something in the “mould juice” had killed the staphylococci.
Fleming immediately began investigating this strange new substance. He identified the mold as Penicillium rubrum and named the substance penicillin.1 He published his findings in the spring of 1929 in The British Journal of Experimental Pathology.2 But a decade later, pharmacologist Howard Florey and biochemist Ernst Chain at Oxford would pick up where Fleming left off. Alongside a USDA lab in Peoria, Illinois, the pair would develop penicillin into a life-saving drug and usher in the era of antibiotics.
This is the kind of science story everyone likes. One of serendipity and accidental discovery; a chance observation that changed the world. But is it true?

For decades, scientists and historians have puzzled over inconsistencies in Fleming’s story. For starters, the window to Fleming’s lab was rarely (if ever) left open, precisely to prevent the kind of contamination that supposedly led to penicillin’s discovery. Second, the story is strikingly similar to Fleming’s earlier discovery of lysozyme, another antibacterial substance, which also featured lucky contamination from an open window. Third, Fleming claimed to have discovered the historic culture plate on September 3rd, but the first entry in his lab notebook isn’t dated until October 30th, nearly two months later.
Last, and most important: penicillin only works if it’s present before the staphylococci. Fleming did not know it at the time, but penicillin interferes with bacterial cell wall synthesis, which only happens when bacteria are actively growing. Visible colonies, however, are composed mostly of mature or dead cells. By the time a colony can be seen, it is often too late for penicillin to have any effect. In fact, the Penicillium mold typically won’t even grow on a plate already filled with staphylococcus colonies. For years, scientists have attempted to replicate Fleming’s original discovery. All have met with failure.
Thus, it’s difficult to reconcile Fleming’s story with these historical and scientific discrepancies. Did he misremember events from 15 years earlier? Could he have fudged the details to make for a more compelling narrative? Or, might Fleming’s experiment have been subject to an unusual confluence of chance events unbeknownst even to him?
Speculation about how Fleming discovered penicillin is of little consequence compared to its practical impact. However, science is about evaluating evidence and moving closer to the “truth.” As we near the 100th anniversary of penicillin’s discovery — which undoubtedly will encourage even greater repetition of the story — it’s in this spirit that we must scrutinize the story’s veracity.
The historical and scientific data are limited and often contradictory. Nevertheless, several scientists and historians have worked hard to piece together what facts are certain and fill the gaps with their most probable guesses. The result is a range of competing theories, each attempting to explain what really happened in that St. Mary’s Hospital laboratory in the summer of 1928.

{{signup}}
Fleming’s Account
The story of Fleming’s discovery of penicillin is primarily based on this passage from his 1929 paper:
"While working with staphylococcus variants a number of culture-plates were set aside on the laboratory bench and examined from time to time. In the examinations these plates were necessarily exposed to the air and they became contaminated with various micro-organisms. It was noticed that around a large colony of a contaminating mould the staphylococcus colonies became transparent and were obviously undergoing lysis (see Fig. 1)."
“Fig. 1” refers to a “Photograph of a culture-plate.” It shows separate, well-grown staphylococcus colonies around 2-4 mm in diameter spread across most of the plate’s surface. But on one edge, a large mold colony of about 20 mm in diameter, plus a secondary satellite colony, is clearly visible. This is labeled “Penicillium colony.” Surrounding it is a zone of about 20 mm in which the staphylococcus colonies are either not visible or have become semi-transparent ghosts. Those nearest to the mold are smaller than the rest, only 0.4 to 0.8 mm, while those towards the periphery are a bit larger, 0.8 to 1.7 mm. Fleming has labeled these “Staphylococci undergoing lysis.” Later, Fleming and his colleagues would claim that this was the original contaminated plate from which Penicillium was first isolated.
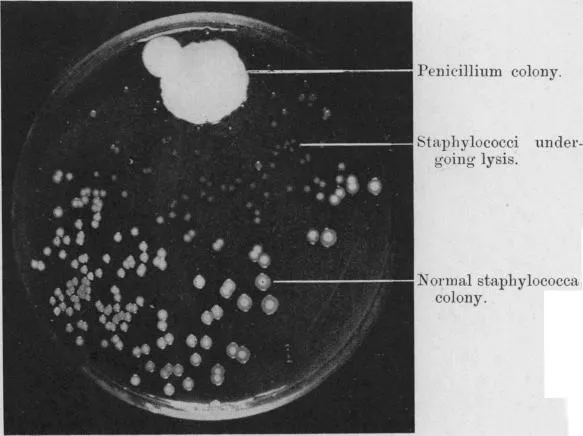
On its face, this seems simple enough. Everyone knows that penicillin destroys bacteria, and Fleming observed staphylococci seemingly being destroyed by a mold that produced penicillin.
However, upon closer reading of Fleming’s 1929 paper, it becomes clear that a great deal of work was either omitted or inadequately described. There is, for example, no description of the type of culture medium used; whether or not the plate had been incubated; how long it had been on the bench; and, most important of all, what species of Staphylococcus was being studied.
When publishing a scientific paper, scientists are expected to include a detailed description of their methods alongside their results. Like a recipe, these methods should clearly and comprehensively describe the materials used and the steps taken so that other scientists can replicate the experiment. And while incomplete or poorly-described methods are a perennial problem, the omission of these key experimental details (even in a report on an accidental discovery) is surprising.
This became a problem when, as interest in penicillin grew, other investigators tried to repeat Fleming’s discovery. In 1944, Margaret Jennings (who later married a long-time colleague and penicillin researcher, Howard Florey) spread purified penicillin onto plates of fully grown staphylococci. This should have had a more potent effect than Fleming’s pictured in Figure 1, which was allegedly produced only with the crude “mould juice” from an accidental contaminant. Jennings, however, observed no visible change.
In 1965, the pathologist W.D. Foster attempted a similar experiment using penicillin crystals dropped directly onto staphylococcus colonies, creating “astronomical” concentrations within their vicinity. But still, the colonies remained unaffected.
Other attempts at replication called into question whether the mold could have even grown on a plate full of staphylococci. Pharmacologist D.B. Colquhoun claimed that, in 1955, he found that Penicillium mold refused to grow on a plate already full of staphylococcus colonies. Or, that, if it did, it produced no visible effect on the colonies. He could, however, see an effect if the sequence of events was reversed: if the mold was allowed to grow for several days first, and the staphylococci was later inoculated onto the plate.
Although these failures are hard to reconcile with Fleming’s account, they are in line with what we now know about the biology of penicillin.
In 1940, the physician A.D. Gardner, researching alongside Florey, peered into his microscope to examine how penicillin affected individual bacterial cells. Surprisingly, adult cells seemed to be largely unaffected; however, when they divided, the young cells grew “as immense swollen filaments.” Like party balloons, they elongated and expanded, then popped.
“The morphological changes,” observed bacteriologist J.P. Duguid in 1946, “suggest that penicillin in these concentrations interferes specifically with the formation of the outer supporting cell wall, while otherwise allowing growth to proceed until the organism finally bursts its defective envelope and so undergoes lysis.” At the time, this was largely speculation. Not much was known about the biology of bacterial cell walls. But after a decade of study — motivated in no small part by a desire to understand how penicillin worked — this hypothesis has largely been proven correct.

The bacterial cell wall is a rigid, mesh-like structure composed primarily of peptidoglycan, a large macromolecule consisting of small subunits cross-linked by specialized enzymes called transpeptidases. The job of the cell wall is to maintain the cell’s shape and keep it from absorbing too much water. If the outward pressure from the cell’s contents becomes too great for the delicate cell membrane to contain, it bursts, spilling the cell’s innards. The cell wall, like a heavy-duty bicycle tire around a rubber inner tube, helps to resist this pressure, protecting the cell from mechanical stresses both inside and out.
Unlike a bike tire, however, cell walls need to be able to grow with the cells they enclose. To accommodate increasing cell size, bacteria are continuously breaking and rebuilding the peptidoglycan mesh. This is where penicillin comes in. Because penicillin has a similar chemical structure to a peptidoglycan subunit, it can bind to the transpeptidases that complete the final step in cell wall biosynthesis.3 When this happens, penicillin forms a covalent bond in the transpeptidase’s active site, irreversibly inactivating the enzyme. As it grows, the cell continues to disassemble its cell wall, but without the use of its transpeptidases, it can no longer rebuild it. Over time, the cell wall weakens and eventually bursts.
This explains why Jennings and others couldn’t replicate Fleming’s contaminated plate. A mature colony is mostly composed of adult or dead cells. These cells are unaffected by penicillin because they aren’t actively growing, and so aren’t actively breaking and rebuilding their cell wall. As a result, penicillin doesn’t cause mature cells to lyse, and the colony’s overall appearance doesn’t change. But if the penicillin is present before the staphylococci, it prevents the bacteria from growing and dividing, or they do so much more slowly. When that happens, they don’t form visible colonies. Thus, penicillin does not dissolve fully grown colonies, as Fleming had initially assumed, but inhibits their growth from the start.
The difficulty replicating Fleming’s discovery is frustrated by the ease with which it’s possible to “rediscover” penicillin by reversing the order of growth. By first growing Penicillium mold until it becomes a large colony, then seeding the plate with staphylococci, the result is indistinguishable from Fleming’s original plate. However, no trained scientist would intentionally use a culture plate visibly contaminated with a large mold — and certainly not an expert bacteriologist like Fleming.4
There are no contemporary records to corroborate the story that Fleming discovered the contaminated culture plate when he returned from holiday on September 3rd: no lab notebook records, calendar notes, diary entries, or any letters. In the 1929 paper, the figure is simply labeled, “Photograph of a culture-plate.” The only evidence we have stems from recollections by Fleming and colleagues years later, after penicillin was recognized as a runaway clinical success. Fleming himself described the Figure 1 plate as the “original culture plate” in a 1944 paper. Yet, he also included the disclaimer that “after a lapse of fifteen years it is very difficult to say just what processes of thought were involved.”5
The earliest recorded mention of the mold and penicillin is an experiment written in Fleming’s lab notebook dated October 30th, 1928 — nearly two months after he purportedly found the culture plate. Curiously, it does not describe the chance discovery of a contaminant, but a carefully constructed experiment that suggests Fleming had already spent some time isolating and characterizing the mold. In it, Fleming used the reversed-sequence culturing method: first, placing a mold spore on the plate and letting it grow into a large, penicillin-producing colony, then inoculating several pathogenic species of bacteria, including staphylococci, near the mold.
On October 30th, Fleming recorded the results: the mold affected a whole host of pathogens, including staphylococci, which could not grow near the mold. It’s a fine experiment, but it’s clearly not the discovery of an accidentally contaminated culture plate. This raises the question: What was Fleming doing for the previous two months, and if he was working with penicillin, why didn’t he bother recording any of it?
For decades, these scientific inconsistencies and experimental failures have haunted the story of penicillin’s discovery. Amidst the incontrovertible Nobel Prize-winning scientific and clinical success of penicillin — and without a plausible alternative — the doubters kept quiet. At least, most of them.
Ronald Hare’s Theory (1970)
In 1964, the bacteriologist Ronald Hare took up the puzzle of penicillin’s origins. After examining old lab notebooks and conducting experiments of his own, he would conclude that “the history of both the culture plate and the mould itself must have been very different from what had previously been thought to be the case.” Hare published his own theory on penicillin’s discovery in his 1970 book, The Birth of Penicillin, and the Disarming of Microbes.
Hare was uniquely positioned to investigate this mystery. Not only was he an accomplished bacteriologist and expert on penicillin, having spent 20 years as a Professor of Bacteriology at the University of London, and the ten years before that at the University of Toronto, where he was largely responsible for planning and building the Canadian Government’s penicillin plant; he started his career in the same department as Fleming at St. Mary’s. In fact, he claims to have been in the laboratory the very day Fleming discovered the now-famous culture plate. (Despite this close professional association, however, Hare claims to have played no part in the discovery or original research on penicillin nor to have discussed them with Fleming.)
Although that discovery is now regarded as one of the most significant scientific events of the 20th century, Hare admits that, at the time, it made little to no impression on him or any of his colleagues. “The rest of us, being engaged in researches that seemed far more important than a contaminated culture plate, merely glanced at it, thought that it was no more than another wonder that Fleming seemed to be forever unearthing, and promptly forgot all about it.”
And yet, Hare had been skeptical from the start that penicillin could have been discovered by simple contamination of a culture plate. It was such a common occurrence in biology laboratories that “if this had been the sequence of events, penicillin would probably have been discovered while Fleming was still a child.”

After retirement, Hare took up the penicillin question. He began by attempting to replicate Fleming’s discovery. He seeded an ordinary culture plate with staphylococci, incubated it until colonies were visible, then placed a few mold spores on the surface. As the microbiologists before him had observed, the mold refused to grow. He tried coaxing the mold’s growth by plating it further and further away from any staphylococcal colonies (without deviating too far from the overall appearance of Fleming’s Figure 1).
With this approach, he was finally able to get the mold to grow and produce penicillin, but still the staphylococcal colonies were unaffected. “No one looking at such a plate could possibly guess that a powerful antibacterial substance was emanating from the mould.” If, however, he reversed the order and plated the mold before the staphylococci, he could get a result “almost indistinguishable from that of Fleming’s original plate.”
Vexed, Hare reevaluated the evidence. He had shown the mold couldn’t have contaminated the plate after the staphylococci because the mold wouldn’t grow (or, if it did, the penicillin wouldn’t affect the staphylococcus colonies). He assumed that the contamination couldn’t have occurred before the staphylococci (though that reliably recreates the plate pictured) because no bacteriologist would knowingly use a contaminated plate.
What if the mold contaminated the plate at the same time, or within a few hours, of when it was seeded with staphylococci? And what if the staphylococci’s growth had been paused (somehow) until the mold colony had matured? To Fleming’s eyes, he would have assumed he had inoculated staphylococci onto a contamination-free culture plate. Yet, with the staphylococci’s growth delayed, the mold would have had time to fully develop into a large, penicillin-producing colony. When the staphylococci’s growth was restarted, it would be growing in effectively the same conditions as if it had been plated after the mold had grown.
Hare knew just the thing that could arrest the staphylococci’s growth: low temperature. Staphylococci grow most rapidly at 98.6 °F (37 °C). As a human pathogen, it has evolved to grow optimally at human body temperature. This is why microbiologists incubate culture plates: to speed up their growth into visible colonies. The lowest temperature at which any staphylococci growth occurs, and then only very slowly, is around 53 °F (12 °C). The Penicillium mold, on the other hand, prefers to grow around 77 °F (25 °C) but is not greatly affected by temperature ranges.
It therefore seemed possible that penicillin could have been discovered as described in the original 1929 paper, but with the addition a few details Fleming was unaware of: first, the inoculation of staphylococci and contamination by mold occurred at the same time; second, Fleming forgot6 to incubate the plate; and third, the lab’s room temperature was low enough for long enough for the mold to grow and produce penicillin before the staphylococci began to grow.
To test this theory, Hare simultaneously inoculated a culture plate with both staphylococci and Fleming’s mold and left it on his benchtop. The weather that day was cold, wet, and stormy, and the temperature was relatively low: 61 to 65 °F (16.1 to 18.3 °C). As expected, the staphylococci grew more slowly than they would have in an incubator, and only tiny transparent colonies were visible on the third day. The mold, however, grew much more prolifically, and a tiny colony was visible after just 48 hours, growing to 10 mm by the fourth day.
By the end of the fifth day, Hare had rediscovered penicillin. The result was practically indistinguishable from the photo in Fleming’s original paper: in a ten millimeter zone around the mold, the staphylococcus colonies were small and transparent, while those outside the zone were larger and opaque. Many experiments later, Hare found that penicillin could reliably be rediscovered in this manner so long as the temperature was kept below 68 °F (20 °C) for four or five days.
This is where Hare the scientist had to become Hare the historian. Was the temperature in Fleming’s laboratory low enough for him to discover penicillin at the end of July or the beginning of August in accordance with the timeline of his canonical story?7
Hare searched the records at the Meteorological Office for the maximum and minimum shade temperatures from the beginning of July to the end of September, 1928. In the weeks before Fleming left on holiday there was a heatwave; from July 10th to 27th, there were highs in the upper 70s and 80s. At these temperatures, the staphylococci would have grown too quickly.
However, on the 28th, the heatwave ended and was quickly replaced by a cold snap. For the next nine days, the maximum temperature only exceeded 68 °F on two occasions, and not by much. It was a slim window, in which the temperature in Fleming’s laboratory would have been low enough. But it coincided perfectly with Fleming’s holiday.
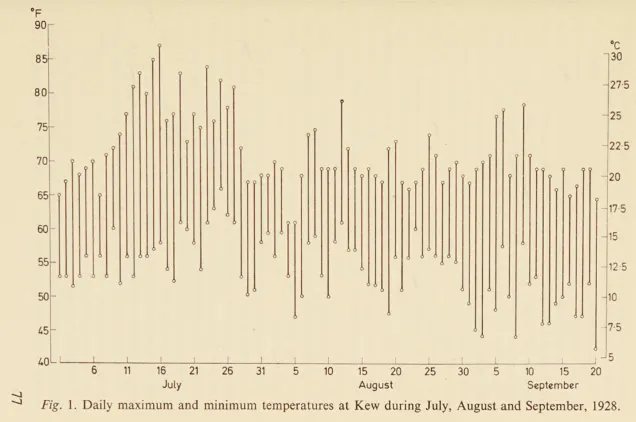
Hare’s theory relies on a triple chance of unlikely events. First, the penicillin-producing Penicillium mold landed on Fleming’s culture plate; second, Fleming failed to incubate the plate; and third, the temperature was low enough for the five days required to favor mold growth.8 “Had only one link in this chain been broken,” Hare writes. “Fleming would have missed his opportunity.”
Hare himself concedes that the combination of these contingencies seems exceptionally unlikely. The original story of chance discovery, on its own, was one of serendipity and good fortune. His theory required an additional layer of meteorological luck on top of chance contamination. “Far from the phenomenon that led to the discovery being a comparatively common event that had previously escaped detection, it must be so unusual an occurrence that it is doubtful whether it can have happened very often since bacteria were first cultivated in the laboratory.” Yet, however improbable it may seem, to quote Sherlock Holmes: “When you have eliminated all which is impossible, then whatever remains, however improbable, must be the truth.”
Robert Root-Bernstein’s Theory (1989)
Hare’s theory is based on the assumption that the Figure 1 plate was indeed the source of the original contamination. It also overlooks the two-month gap between when Fleming allegedly noticed the contaminated plate and recorded the first penicillin experiment. These details, however, form the foundation of a competing theory on penicillin’s origins — that belonging to Robert Root-Bernstein, Professor of Physiology at Michigan State University.
Root-Bernstein described his theory in his 1989 book, Discovering: Inventing and Solving Problems at the Frontiers of Science.9 It’s an ambitious and unorthodox book, structured as a seminar between six characters discussing creativity and the scientific process. Each character represents various points of view on the sciences and scientists and, along the way, they also discuss the important chronological and methodological idiosyncrasies of Fleming’s discovery.
Root-Bernstein’s theory is argued and defended by the character Imp (real name Ernest; apparently a stand-in for the author himself). After summarizing the key details of Hare’s theory, Imp focuses on the two-month gap. Fleming supposedly discovered the Fig. 1 contaminated plate when he returned from a holiday on September 3rd, but the first lab notebook entry about Penicillium and penicillin wasn’t written until October 30th. As described earlier, that entry does not record the discovery of a contaminated plate but a planned experiment in which the Penicillium mold was first isolated and tested against several bacteria, including staphylococci.
But why, Imp wonders, if Fleming already had that beautiful culture plate which so perfectly illustrated the staphylococci-killing power of penicillin, he would wait two months to record the finding? And why do so in the context of another experiment? Furthermore, this plate still exists within the British Museum. That means Fleming had to fix it with formaldehyde relatively soon after he found it. But if he thought the plate was important enough to preserve, why didn’t he note its discovery at the time?
It wasn’t in Fleming’s character to procrastinate. According to his research scholar, Merlyn Pryce: “[Fleming] didn’t confine himself to observing, but took action at once. Lots of people observe a phenomenon, feeling that it may be important, but they don’t get beyond being surprised — after which, they forget. That was never the case with Fleming.” So then, what was he doing for two months?
It’s in this context that Imp (Root-Bernstein) grounds his belief that Fleming’s discovery wasn’t the serendipitous chance of lore — at least, not completely. Instead, he proposes that Fleming wasn’t running a staphylococcus experiment when he discovered penicillin; he was looking for new sources of lysozyme.
Fleming had a long-standing professional interest in antibacterial substances. His most important discovery before penicillin was the lysozymes, enzymes found in various bodily fluids (e.g., tears, saliva, and egg whites) that break down the cell walls of bacteria. He had also studied the antibacterial properties of mercuric chloride and bacteriophages.
Between 1922 and 1928, Fleming’s team tested anything they could get their hands on: human mucus, tears, sputum, and blood; the eggs of dozens of fish and bird species; tears collected from horses, cows, hens, ducks, geese, and fifty other species from the London Zoo; earthworm and snail slime; large numbers of vegetables and flowers. They continued to publish on lysozymes into the 1930s. “Is it too much to suggest,” Imp asked, “that he also examined any fungus that happened to come his way?”
If we assume that Fleming was engaged in a systematic search for new sources of lysozyme, we can now reasonably fill in the gap between September 3rd, when he first spots the mold, and October 30th, when he first records the Penicilliumexperiment. Root-Bernstein’s theory about the discovery goes like this:
First, Fleming begins by finding the mold, which may or may not have been on a staphylococcus plate. In the paper, Fleming only says that he found it at the time he was “working with staphylococcus variants.” Either way, the plate is not enough to incite a “Eureka!” moment, as the canonical version of the story suggests.10 Instead, like the hundreds of other unusual samples he’s tested, Fleming transfers the mold to a new culture plate, gives it a few days to establish itself, and then runs a routine experiment to test for lysozyme activity. He finds that it weakly affects a lysozyme-sensitive strain. Not terribly interesting — not even worth recording11 — but it warrants a follow-up.
A short time later, the Root-Bernstein theory goes, Fleming prepares a second experiment. After growing the mold for five days into a robust colony, he adds various lysozyme-sensitive and -resistant species to the plate, including Staphylococcus. This time, he records the results because (surprise!) the mold affects the lysozyme-resistant staphylococci. This experiment, whose results are recorded on October 30th, is exactly what it appears to be within the context of Fleming’s notebooks: “the first penicillin experiment — the first recognition by Fleming that he’s dealing with something unexpected and exciting!”
Yet, if this is the true sequence of events, why didn’t Fleming record it as such in his 1929 paper? “The logic of presentation rarely corresponds to the logic of discovery,” said Imp. Few scientists actually document the chronological sequence of events that led to their discovery. “Just imagine for a moment trying to write into a research paper the account I’ve just given,” said Imp:
"While looking essentially randomly for organisms producing lysozyme, a common but unidentified mold was isolated from the air of the laboratory. Initial experiments showed that the mold appeared to have lysozyme activity, so controls were set up, including staphylococci, which I just happened to have been working on at the time. Much to my surprise, the mold had unexpected properties, so I was now forced to further characterize and identify the mold …This subsequent research conclusively demonstrated that the product of the mold was not lysozyme, but rather a new substance having the following characteristics …"
It’s too circuitous and indirect for a scientific report. Better, instead, to start with the mold lysing the pathogen, because that was the important and novel observation.
Under this theory of events, the Figure 1 plate also becomes exactly what it appears to be in its proper context: an illustrative example of the fact that penicillin-secreting Penicillium can kill staphylococci. Not, as the story is typically told, the original contaminated plate. Reading further in the paper, similar examples are included as Figures 3 and 4, which illustrate other properties of penicillin.
Fleming still could have shown his colleagues a contaminated staphylococcus plate on September 3rd, but one which must not have had the telltale “ring of death.” Or perhaps he did pass around the plate that would become the famous Figure 1, but not until several months later, when he was preparing figures for his paper. Hare’s cold snap, too, may have played a role in the mold growing when it did, but it no longer has to coincide with Fleming inoculating his staphylococcus plate.
That Fleming was originally searching for new sources of lysozyme could also explain why he thought the mold contaminated a staphylococcus plate after the colonies had fully grown (which, barring Hare’s theory of simultaneous contamination, should be impossible). Penicillin may not be able to lyse mature colonies, but lysozymes can. Fleming may have assumed penicillin lyses bacteria the same way as lysozymes, and therefore could lyse mature colonies. It’s a conceptual leap, but one made smaller if he was looking for lysozymes in the first place.
Like Hare, Root-Bernstein does not claim his account of Fleming’s discovery is “true,” only that it’s compatible with available data. (Root-Bernstein does not, however, shy away from claiming that his theory is the more likely of the two. “Hare may be a good bacteriologist,” said Imp, “but I question his historical acumen. Dates — you’ve got to pay attention to dates.”)
More important to Root-Bernstein than the specifics of Fleming’s discovery is the fact that it evidences Pasteur’s principle that “chance favors only the prepared mind.” Whether he was experimenting with staphylococci or lysozyme, Fleming kept his mind open to the possibility of discovering new bacteriolytic substances. He often gave the advice, “Never neglect an extraordinary appearance or happening. It may be — usually is, in fact — a false alarm that leads to nothing, but may on the other hand be the clue provided by fate to lead you to some important advance.”12
Fleming’s methods — which included testing strange samples and keeping plates around for longer than he needed them — increased the probability that he would stumble upon something new, and he was mentally prepared to recognize them when he did.
Source of the Mold
The other important aspect of Fleming’s discovery is the source of the contaminating mold. According to the canonical version of the story, the Penicillium floated into Fleming’s lab from an open window. However, no such claim was made in the 1929 paper. In fact, nothing about its source was said until 1945 when Fleming told the writer George Lacken that it had blown through the window from Praed Street.
Why Fleming would say this is a mystery. He had no evidence that was the case, and as Hare writes, opening a laboratory window is “thoroughly bad bacteriology.” Further, Fleming’s windowsill was often piled high with test tubes and beakers filled with pathogenic bacteria. It would have created quite a scandal should any of these have fallen out of an open window onto the heads of the vulnerable passersby below. Nevertheless, the story gained wide publicity after André Maurois repeated it in his 1959 biography, The Life of Sir Alexander Fleming. Maurois repeatedly referred to “the mysterious mould from Praed Street,” and “the spore carried by the wind.”
Fleming himself seemed unsure of its origins. In a 1946 speech at the Mayo Clinic, he claimed ignorance of its source, “a mould spore coming from I don’t know where, dropped on the plate.” But in another speech in Edinburgh that same year, he claimed, “penicillium had dropped through the window.”

In Hare’s account, the Penicillium came not from the window but the stairwell. In Hare’s 1970 book, he notes how immediately below Fleming’s laboratory, in the same turret of the building, was a mycology lab run by C.J. La Touche. La Touche studied how molds can trigger asthma. He spent much of his time swabbing carpets and curtains in homes inhabited by asthma patients and growing strange and uncommon species of mold from these samples. In the process, he had acquired quite a large collection. But, as Hare recalls from his time working in the same building, La Touche’s lab wasn’t equipped with the fume cupboards or hoods that most mycologists used to prevent mold spores from contaminating the air. As a result, the air in La Touche’s was liable to be full of floating spores, waiting to be carried wherever the breeze might take them.
Both La Touche and Fleming’s labs had doors that opened to a shared stairwell. It is therefore likely that the spore that contaminated Fleming’s plate had originated in La Touche’s laboratory, having traveled out the door of La Touche’s lab, up the stairs, and into Fleming’s. Yet even if none of La Touche’s spores took this journey, at the very least, La Touche himself did: Fleming cites La Touche as the mycologist who identified the mold as Penicillium.
Theories, Plausible and Implausible
As we approach the 100th anniversary of Fleming’s discovery of penicillin, no definitive answer to this mystery has emerged. Other scientists have proposed a handful of additional theories,13 some of which rely on events even less likely than Hare’s, but Hare and Root-Bernstein’s seem to be rooted in the most solid evidence.
For what it’s worth, I believe Root-Bernstein’s theory. Hare’s is scientifically possible, but it relies on an exceptionally improbable sequence of events requiring luck on the order of picking the correct Powerball numbers three (or more) times in a row. Root-Bernstein’s is simpler and more in tune with the psychology and habits of working scientists. It only requires accepting that Fleming and his colleagues misremembered the identity of an unrecorded and, admittedly, forgettable culture plate from 15 years earlier — which seems entirely plausible. Occam’s razor suggests the simplest explanation is usually the best one, and that is Root-Bernstein’s.
The story of Fleming’s discovery of penicillin is not just an interesting historical anecdote; it’s held up as a prime example of momentous inventions discovered by accident. It looms large among discussions about the nature of discovery and how to encourage it. But if Root-Bernstein’s theory is true, and Fleming actually found penicillin while searching for new lysozymes instead of while doing an unrelated staphylococcus experiment, can it really be called an accident?
Of course, penicillin isn’t lysozyme, and a deliberate search for one thing that results in finding something else can still be deemed accidental. Yet, finding a new kind of bacteriolytic substance while looking for a different kind of bacteriolytic substance seems, at least, to be one of a lesser order. Fleming may have been fishing for lysozyme, but his methods — testing strange contaminants for the ability to lyse other microbes — formed a net that, sooner or later, was bound to catch something else.
Root-Bernstein’s theory thus turns penicillin from an example of an “accidental discovery” into one that reflects what the computational biologists Itai Yanai and Martin Lercher have described as an “evolutionary process” in scientific research. In this conception, research isn’t a linear march, but an evolutionary tree, full of once-promising branches that proved fruitless and unexpected offshoots that led to new discoveries. Such an evolutionary history, they argue, “is generally obscured in the resulting scientific publication,” which favors neat teleology.
Fleming’s 1929 penicillin paper may have been written as a linear process, but that’s almost certainly not how the discovery occurred. And by eliminating these complicated twists and turns, Fleming inadvertently obscured what may be one of the most important lessons in scientific history: how combining a meticulous research program with the openness to branch out into new directions led him to Nobel Prize-winning success. Neither rigid plans nor the winds of chance are enough on their own; discovery requires both.
Ultimately, whatever sequence of events actually occurred, what mattered was that Fleming was primed to make the key observation when chance presented it and jumped on what he saw. The rest is history.
{{signup}}
{{divider}}
Kevin Blake is a scientific editor at Washington University in the Division of Laboratory and Genomic Medicine. He writes about microbiology, bioinformatics, and evolution.
Header image by Ella Watkins-Dulaney.
Cite: Blake, K. “The Penicillin Myth.” Asimov Press (2025). https://doi.org/10.62211/04kq-22ub
Further reading:
- Hare, Ronald. 1970. The Birth of Penicillin, and the Disarming of Microbes. London: Allen & Unwin.
- Root-Bernstein, Robert Scott. 1989. Discovering: Inventing and Solving Problems at the Frontiers of Scientific Knowledge. Cambridge, Mass.: Harvard University Press.
- Macfarlane, Gwyn. 1984. Alexander Fleming: The Man and the Myth. Cambridge, Mass.: Harvard University Press.
- Rosen, William. 2018. Miracle Cure: The Creation of Antibiotics and the Birth of Modern Medicine. New York, New York: Penguin Books.
Footnotes
- Later, it would be re-identified as P. notatum, then P. chrysogenum, and most recently, P. rubens.
- The reasons why Fleming did not pursue penicillin research further has been attributed to a multiple technical, institutional, and personal reasons, the history of which could be an essay in its own right.
- Because of how they were discovered, transpeptidases were originally named “penicillin binding proteins” (PBPs). However, binding to penicillin is not their normal function, to my great confusion as a microbiology undergraduate.
- In 1927, Fleming was the “obvious choice” to write the chapter on staphylococci in the large nine-volume System of Bacteriology. This was generally referred to as “the Bible” in bacteriology labs because it was supposed to contain the whole of existing knowledge.
- Casting further doubt, Fleming stated in that same paper, that “what had originally been a well-grown staphylococcal colony was now a faint shadow of its former self,” but we now know that is not possible. Fleming’s colleagues have also claimed that was the plate Fleming showed them in September. Yet, here again, no records verify this.
- Not incubating the plate may have been done intentionally. Fleming was known to make “agar art,” pictures on culture plates done by “painting” with colorful bacteria. Though incubation is standard practice because it grows bacteria quickly, it can affect how vivid the colors of the colonies appear. Some suspect Fleming may have deliberately failed to incubate his staphylococci just to see what would happen.
- This was before climate control systems and LEED-certified buildings. Though a gas fire kept Fleming’s laboratory warm in the winter, for the rest of the year the temperature could fluctuate violently from the heat of the sun and cold winds from the east and south.
- Actually, four unlikely events combine. Fleming later claimed that he had already discarded the plate onto a pile in a Lysol bath, and it was only later, while talking with his research scholar, Merlyn Pryce, that he noticed the historic plate, high and dry, and rescued it. Had the pile been cleared away a little sooner, or the antiseptic bath filled a little deeper, then that one-in-a-million plate would have been lost forever.
- I was made aware of Root-Bernstein’s theory, and in turn the entire alternate universe of penicillin theories, by William Rosen’s 2018 book, Miracle Cure: The Creation of Antibiotics and the Birth of Modern Medicine.
- Actually, it has been claimed that, instead of “Eureka!” Fleming muttered the more modest, “that’s funny…”
- As Hare writes, “what mattered most to Fleming was not the recording of his experiments but his performance.” The absence of lab notebook entries, therefore, is not necessarily evidence that he didn’t run any experiments between September 3rd and October 30th. It could just as well have been that none were interesting enough to be recorded.
- Lecture at Harvard University. Quoted in Joseph Sambrook, David W. Russell, Molecular Cloning (2001), Vol. 1, 153.
- Gwyn MacFarlane describes a handful of these in his 1984 book, Alexander Fleming: The man and the myth.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.