A Liver on Ice

On a Saturday night in autumn, an ambulance slid out of a large hospital’s emergency cul-de-sac and silently hit the road. Three of us sat facing each other in the back.
Dr. Johanna Lee,1 in red ear warmers and a waterproof jacket, looked like she was going skiing. “Trudy,” she was saying. “Long time no see! What’s it been, two weeks?” Trudy, a sturdy middle-aged woman with big, no-nonsense blue eyes in sweatpants and an old fleece, was dressed as if she were running out to get the mail. But we were doing neither. We were going out to save a life.
We picked up speed on the highway. It was just before 11:00 pm, and the roads were empty. Our suitcases rattled on the stretcher along with an empty picnic cooler, big enough to hold some fifty cans of Coke. It was impossible to hear each other over the sound of clinking medical equipment, so Dr. Lee and Trudy showed me pictures on their phones. The women’s albums looked the same: food, flowers, healthy livers, diseased livers, pediatric livers, and livers of unusual sizes.
The ambulance didn’t take us to the closest public airport, but to the single lobby of the private charter, deep in the dark nothingness of Middle America. As we waited, we were joined by a young resident named Julia. From there, the four of us were ferried in our shiny black SUV to our private plane, a King Air 350. I felt like the President, walking up an airstair as part of an entourage as midnight approached.
Once inside, I buckled my seatbelt, so close to the pilot that I was practically in the cockpit. No one stopped talking during liftoff. Only I seemed nervous.
As the only non-surgical personnel who would be taking part in the transplant, I had every reason to be. I was simply someone who, in 2022, had reached out to a hospital about observing a transplant while researching the liver’s cultural history in my graduate nonfiction program. Initially surprised when my request was granted, I now know that most of those involved with liver transplants wish the organ got more public attention. That’s what I was there to help with: describing the history and the ethics of its transplantation and the complex procedure itself — the unlikely transfer of a living organ from a brain-dead donor to its hopeful recipient.
Somewhere below us, that very recipient was on her way to the hospital we had just left, where she would be scrubbed clean, strapped down, and sedated. If nothing went wrong, we would return with her new liver before dawn.
After thirty minutes, we landed and were whisked into another SUV. No one on our team had been to this hospital before. On a liver procurement, Dr. Lee explained, you rarely know where you’re going. At the hospital, we were led through hospital corridors to a locker room, where we changed into scrubs purchased from a vending machine.

The operating room was already bustling with people our team didn’t know and would likely never work with again. While technicians laid out rows of surgical instruments, yet others showed Dr. Lee scans or busily prepped buckets, ice boxes, and tubes. For twenty minutes, people came and went, introducing themselves, until someone announced, “He’s here.”
He looked like a peaceful, sleeping man, but, though officially dead, he was still breathing, the rising of his shaved chest accompanied by the huff of a ventilator.
John had been found unresponsive after experiencing a stroke. Within days, he had been declared brain dead. Otherwise, his body was healthy, the perfect candidate for organ donation. In his waking life, however, John had technically never signed up to be an organ donor.
There are two models for giving organs. The first, common in Europe, relies on “presumed consent.” Unless you explicitly opt out, your healthy organs belong to society, and you need special permission to be buried with them. In the U.S., the model relies instead on “donation.” Legally, a donated organ is considered a “gift” that an individual makes to another, and the decision to make that gift is entirely the donor’s own.
Where we were in the Midwest, the Anatomical Gift Act states that if someone is not alive to give consent, you must get it from whoever has power of attorney over them.2 When John died, no one claimed his body. And when the organ procurers called the people listed in his medical file, they claimed never to have heard of him. In this case, the law grants “any other person authorized or under legal obligation to dispose of the body” the power to sign off on donation. In John’s case, this was the coroner.
{{signup}}
John’s name went into the database of the Organ Procurement and Transplantation Network (OPTN), the non-profit that matches every transplant in America. John’s match came from his region’s procurement program, the group that owns the King Air we flew on and employs Trudy as an Organ Recovery Coordinator. She accompanies transplant surgeons like Dr. Lee to manage procurement logistics, from assessing organ eligibility3 to overseeing their handling and transportation.
A procurement can be an emotional operation. Some families write letters to be read aloud before the first incision, while others request that the deceased’s favorite song be played. Dr. Lee remembers an operating room where they watched a three-minute montage of a young girl set to music. Forty people from different states were there to procure her organs, a different team for each. By the end of the video, everyone was sobbing.
There were no such gestures for John. A transplant nurse recited his name, date of death, blood type, and confirmed that the coroner had authorized the donation. If science is truly in the process of replacing religion, then we might call this his eulogy. After the recitation, Dr. Lee led us in a moment of silence, as she does before every procurement.
When the silence broke, a nurse led me to John’s head, where I stood on a step ladder and watched from above. His belly stretched below me, yellowed with iodine and glossy with anti-bacterial film. By then, his face had been tented over with a blue shroud. Dr. Lee and Julia were transformed as well. Dr. Lee’s casualness had shifted to an effortless concentration. Julia, who had seemed young and bird-like, now moved authoritatively as she lowered a mounted light over John’s chest.
Before Dr. Lee began, she asked me if I had ever seen anything like this. She probably wanted to know if I’d be squeamish or likely to faint at the sight of blood. I told her I had performed thousands of surgeries on mice as a research technician, which reassured neither of us. But with the woman who needed this liver already checking into the hospital, there was no time for hand-holding.
A liver procurement begins with a buzz. Using an instrument called a bovie, a narrow wand whose electrical current cuts skin while sealing the bleeding soft tissues beneath, Dr. Lee made the first incision, from collarbone to pubis. Seconds later, the room filled with the smell of burning hair and a fleshy aroma that only surgery veterans feel comfortable comparing to barbecue. The cutting revealed layers: first, something fluffy and white, like the inside of a cattail, then glistening, yellow fat. Above the belly button, Dr. Lee carved a horizontal line, forming a crucifix. Flaps of skin were pulled back with metal clips, and the inside of the abdomen rose into the light, still moving.
Now came the first true trial of the evening — not of the surgeon’s skill, but of the liver. Non-invasive scans can tell doctors some things about the quality of an organ, like whether it carries a large tumor. But those scans cannot say whether the organ is healthy enough for a transplant; that is a secret only a trained human eye and hand can grasp.
Dr. Lee passed her gloved hands gently over the liver. Despite all the years I’d spent thinking about it, this was my first time seeing one in the flesh. The human liver is huge — a great rust-colored wedge, with a hint of purple in its shiny surface. Partly concealed by the rib cage, it spanned the upper right half of the abdominal cavity from flank to diaphragm.
Dr. Lee went through her checklist. A good liver, she had told me on the ambulance ride to the airport, should feel “like a stingray”: smooth, supple, and soft. She observed the liver’s edges, which should be sharp and defined. Based on what she knew of the height and weight of the woman waiting back at the hospital we had left, she had to judge if this liver would be a good fit — literally, if it would fit inside her.
If John’s liver were found to be bumpy, engorged with fat, too large, or in any way diseased, the whole endeavor would end right there, and the woman would have to drive home and return to the waitlist.
Dr. Lee took photos of the liver on her iPhone (so, this is where all the photos were coming from) and sent them to the chief surgeon back west, Dr. Gibbs, who, if all went well, would be implanting this liver.
After his okay, there was no time to waste. Across state lines, he would begin his half of the surgery momentarily so that the liver could proceed speedily from the plane into its new body.
With quick, small strokes, Dr. Lee and Julia began the part of the process called the “warm dissection” (so called because the donor is still warm). This step involved separating the liver from what had connected it to the body since birth. Dr. Lee gently worked these attachments free. If she were to nick an artery, the man could bleed out.
“Replaced left,” she said to Julia without looking up. She had noticed an anatomical anomaly where the hepatic artery, which goes from the front of the aorta to the liver, follows a slightly different path. Ten percent of people share this same anomaly. In fact, just over half of us have “normal” liver vessel anatomy — the rest have some kind of variation. Because scans often don’t show this, Dr. Lee is used to feeling around for rogue blood pipes. Once the hepatic artery is located, it is important to keep it in one piece, as it will eventually need to connect, seamlessly, to the recipient’s hepatic artery.
All of a sudden, John’s colon flopped out of his abdomen like a pink boa, great and festive. Using her whole body, Julia wrangled it to her chest, allowing Dr. Lee to find the hidden portal vein, which brings blood from the intestines and spleen to the liver. She tied it with silk string and inserted a cannula — a thick plastic pipe attached to an IV — through which fluid dripped to prevent blood clotting.
A warm dissection can take as long as an hour. While Dr. Lee and Julia worked, some nurses monitored the vitals, some saw to tasks like rearranging the instruments, and others clustered at the edge of the room, passing time until they were needed again.
One nurse asked me if I’d like to come around the table to peek into the chest cavity, where the sternum had been sawed open to expose the still beating heart. “That’s something you don’t see every day,” she said. I shifted around the table and peered into John’s chest, which resembled the compartments of a big jewelry box. I saw the heart, wringing itself out like a rag, slapping against a pair of lungs, the texture of raw burger. Why hadn’t his heart stopped yet? I knew the answer, of course. He was brain-dead.
The concept of “brain death” was developed in the 1960s to solve two problems. The first stemmed from the invention of the ventilator in 1952. Almost overnight, artificially maintained vital functions could defer death for those who might otherwise have perished. While ventilators are undoubtedly helpful in the short term, as when keeping sepsis patients breathing while they recover from respiratory failure, they can also forestall the death of patients with no chance of recovering brain function.

The second was that doctors needed terminology for telling families that, even if their loved ones looked like they were just sleeping, with fingernails that still grew and bodies that still urinated, menstruated, and felt warm to the touch, they were, in fact, “gone.” With the advent of successful transplant surgery, these organs could now be used to help living patients recover. As one medical ethicist put it, “It’s the abortion question at the other end of life.”4
I looked at John, hooked up to machines, his heart still beating and his face, under the sterile tent, still pink. At this point, chatter broke out over the body. Dr. Lee and Julia were taking turns squeezing the lungs, looking for cancer. The scans, Dr. Lee explained, had shown a nodule — a hard bump — inside of one of the lungs. They had to be positive that whatever it was was benign, or the transplant would be called off.
“Got it,” she said. The nodule was clear and no larger than a lemon seed. She passed it to a nurse, who relayed it to the pathology lab.
Now the pace picked up. The staff at the back table rustled bags, tubs, and weighing scales. Dr. Lee freed the aorta — the body’s largest artery — from its attachments, so that it snaked around inside the cavity, still flowing with blood. Julia poked a hole in the gallbladder, and green juice squirted out, caught by a clump of gauze. She flushed the bile ducts with saline, cleaning out whatever remained.
Everyone was getting ready for the most dramatic moment of the entire procedure: the cross-clamp. The liver, before being taken from the body, must be completely free of blood so that in transport, nothing clots and nothing rots. The cross-clamp is when circulation to the liver is severed, and the organ gets completely flushed out.

Dr. Lee tied the lower aorta where it branched into the left and right legs, nicked it, and inserted a plastic tube. She clamped the top of the aorta in the chest so that no blood would pump from the heart or go down into the abdomen. Finally, she nicked the inferior vena cava — the big vein that returns blood from the lower body to the heart.
As John bled out, an orchestrated frenzy kicked in around the body. Surgical staff rushed to the site, simultaneously suctioning the blood into a collecting machine and stuffing his body with crushed ice that turned pink on impact. John’s blood spilled into the canoe of his abdomen until it was just a clear solution, and a gray pallor climbed up his chin, his cheeks, his forehead, until only the top of his head still flushed pink.
In the final act, Dr. Lee cut the cords of the liver away entirely — two veins, one artery, and the bile ducts — and lifted it and all its noodle-like vessels, now as gray and bloodless as John, into a blue tray.
From now on, the liver was holding its breath. Every minute that it wasn’t connected to a body was a minute without oxygen, without blood circulating. It could spend only six to nine hours on ice before it began to decompose. Even as Trudy weighed it (three pounds), triple-bagged it, and put it into the cooler, Dr. Lee and Julia started stripping off their surgical gowns to save time. The pathologist messaged Dr. Lee, right on cue, that the lung bump was not cancerous. Relieved, she texted Dr. Gibbs.
Within minutes, Trudy was on the phone coordinating transportation logistics with the plane, the ambulance, the hospital, and the procurement organization, and we piled back into the black SUV, bound for the airport.
Exhausted, I stared out the window of the plane as Trudy sat silently in the back with the cooler pressed to her knees and Dr. Lee and Julia debriefed.
The pace picked up after we deplaned. This time, the ambulance sirens wailed as we headed back towards the emergency cul-de-sac. “Gotta move aside for the liver,” Dr. Lee said. On arrival, she jumped back into action, grabbing hold of the cooler and taking off through the maze of corridors. Later that morning, she would embark on another fly-out procurement in Michigan and perform two liver transplants in the late afternoon.
Struggling behind, we finally reached the small white operating room, where the chief surgeon was already preparing John’s liver for the sleeping patient, sliced open on the table.
The Transplant
Although the recipient lay freshly prepped on the operating table, the work to make this surgery possible began well before tonight.
Records of successful transplants date back as early as the 6th century BCE, and were often performed using portions of the patients’ own bodies. Modest skin grafts, for example, had been attempted by taking skin from a site near the injury and overlapping it on a wound or burn. Ancient Hindu surgeons documented how to use tissue from the patient’s forehead or cheek to build a new nose.5 In 1597, Gaspare Tagliacozzi, the Italian Renaissance surgeon, documented a similar procedure for reconstructing parts of the face by self-grafting in his treatise De Curtorum Chirurgia per Insitionem.

Grafts between people, however, remained a challenge for centuries. Tagliacozzi explained their frequent failure: “the singular character of the individual entirely dissuades us from attempting this work on another person, for such is the force and power of individuality.” Whether this was a philosophical statement or one that presciently gestured at what we today call “rejection” by the immune system of organs that don’t belong to us, we don’t know.
But even as successful grafts between people remained rare, attempts began between animals and humans. In 1667, Jean-Baptiste Denis performed the first documented xenotransfusion, the transfusion of blood from one species into another. In this inaugural attempt, Denis connected the veins of a lamb to those of a feverish boy, who appeared to recover from his illness.6
Buoyed by his apparent success, Denis tried similar procedures on five more people that year. Three recovered; two didn’t. His experiments were halted after the death of Antoine Mauroy, who had received three separate transfusions. Though Denis was eventually cleared of the murder charge lodged by the dead man’s wife (investigators discovered that she, herself, had poisoned her husband), the damage to the procedure’s reputation had been done. Public outrage prompted the Paris Parlement to ban transfusions in 1670, with similar prohibitions issued in England and by the Vatican. Xenotransfusion wouldn’t be attempted again for another 150 years.

By the 18th century, the idea that the body was constructed of distinct, replicable — and sometimes even replaceable — “parts” took hold. Industrialists were beginning to invent technology that not only imitated bodily functions but sought to improve upon them. The spinning jenny and the power loom became extensions of human hands, bifocals corrected vision, and the thresher muscled through the farmland. A flute-playing automata appeared to breathe, and an anatomically realistic mechanical duck pooped in front of an audience on command. The body seemed an intricate collection of parts whose rigorous study could elucidate new functions.
Physicians like John Hunter were doing exactly that — studying cadavers, mapping out anatomies, and categorizing the workings of every ligament and vessel. If the body were a machine, then replacing an organ should be as straightforward as replacing a screw. This created a market for body parts such as teeth. Dentists extracted them from corpses and bought them from battlefields — most famously Waterloo — to make dentures, or cajoled poor children to have them yanked for a cent to transplant into the mouths of the rich. As this new mindset took hold, hostility towards xenotransfusions diminished, and physicians began to experiment once more.
The 19th century saw a flood of animal-to-human skin grafting.7 An English country doctor grafted frog skin onto a burned boy.8 A French physician attempted to graft dog belly skin onto an ulcerated human hand, failing, he claimed, because the dog wouldn’t keep still. A ten-year-old Chicago girl with severe burns was strapped to a sheep’s belly and housed with the animal until her skin could vascularize into the graft. Unsurprisingly, she died, but the same kind of experiment would be tried again and again, with rabbits, cats, rats, chickens, and pigeons. Teeth from dogs, sheep, goats, and baboons were jammed into human gums or used for dentures. Bits of chimpanzee and baboon testicles were transplanted into elderly men who had lost their “zest for life.” Kidneys from lambs and dogs were transplanted into desperate patients, all of whom died.
Not only were such surgeons missing critical knowledge about biological compatibility and the immune system, but they also lacked the tools and skills necessary to connect the organs they were trying to transplant to their recipients. Skin grafts worked because skin is a bit like a tree — the vessels are small and those of the recipient can branch out into the grafted patch, restoring blood flow over several days with little extra encouragement. But when transplanting whole organs, doctors can’t wait around for arteries and veins to grow toward each other as the patient bleeds out. They needed to connect the organ promptly to the patient manually, with a technique called anastomosis. The medical sutures of the time were too thick, and the needles too large, to avoid damaging delicate blood vessels. To overcome such challenges, the world needed a skilled seamstress.
Surgeons had long learned sewing skills from their mothers, sisters, and wives. But in 1901, surgical trainee Alexis Carrel’s mother sent him to someone more gifted, Marie-Ann Leroudier, embroideress of Paris’s high society. Leroudier knew how to sew one-handed and how to be efficient with her thread. She had Carrel practice on cigarette papers, learning how far to push in a needle and how to feel when this delicate material was about to tear, buckle, or give way. Using her methods, he learned how to pierce just a sliver of a blood vessel’s wall, lowering the risk of fatal clots. Although Leroudier doesn’t appear in Carrel’s or most surgical biographies, her techniques are still in use today.

Like his predecessors, Carrel began practicing these skills on animal limbs and organs. Working with collaborator Charles Guthrie, he experimented with transplanting thyroids, kidneys, and hearts. In one especially bizarre experiment, he sewed a dog’s heart to a second dog’s neck, where it beat for two hours. He swapped the legs of white and black dogs, constantly practicing his sutures. As with earlier surgeons, however, his experiments typically ended in the death of his patients. He did notice, however, a curious pattern: Transplants between related animals led to longer survival times than those between unrelated pairs.
Carrel was still piecing together these observations when, in 1908, a desperate father — a surgeon familiar with Carrel’s work — pleaded for his help to save his dying infant using a direct blood transfusion between him and his child. The baby had a rare condition called melaena neonatorum, where blood spurted from her nose, mouth, and anus. Although Carrel hadn’t directly applied the ABO blood-typing discovered in 1901 to his experimentation, he hadnoticed the better odds of survival he’d seen in related dogs. Carrel connected the father’s blood vessels to those of his baby using the anastomosis technique he’d been practicing on dogs. The girl revived, turned pink, and cried. It was the first successful blood transfusion of its kind, and its success laid the groundwork for overcoming transplantation’s next great hurdle — immune response.9
Carrel’s work on vascular suturing (which earned him the Nobel Prize in 1912) culminated in Joseph E. Murray’s first successful kidney transplant in 1954. In this daring procedure, the recipient received a kidney from his twin, who had an immune system compatible enough to avoid severe rejection. By 1966, twenty-three identical twin pairs exchanged much-needed kidneys.10 Surgeons were getting lucky with twins, but it wasn’t until the 80s, with the advent of the immunosuppressant cyclosporine, that non-identical recipients could not only survive the surgery but thrive long after.
Immune rejection threatens all transplants, but each organ also has its specific idiosyncrasies. When it comes to the liver, for example, liver disease impedes clotting, which is essential for any successful surgery. Dr. Thomas Starzl found this out in 1960, when performing the first human liver transplant on a boy named Bennie, born with biliary atresia, a disease in which the lack of functional bile ducts makes it impossible for the liver to eliminate the body’s wastes. By the age of three, Bennie was already dying of liver failure.
Desperate to save their son, Bennie’s parents agreed to the last-resort transplant when Starzl’s team acquired a suitable donor organ. Despite having successfully completed the surgery on 200 dogs, the task was even more challenging than they had imagined. It would take several hours just to make the first incision into Bennie’s abdomen. Bennie was so ill that the blood pressure between his liver and colon was sky-high. His liver was covered in scars, and his blood wouldn’t clot.
This, they would learn, was a common obstacle when operating on people with liver failure. Because the liver makes the proteins critical to clot formation, its failure means that the patient keeps bleeding. Even today, liver transplants can carry unique surgical risks because these patients often arrive at the operating room severely weakened, their blood struggling to clot, which hampers their ability to handle the trauma of major surgery.
Startzl’s patient, Bennie, bled to death despite the team’s desperate attempts to stop the hemorrhaging. In his memoir The Puzzle People, Dr. Starzl remembers Bennie’s body being closed, and then wrapped in a white sheet after “being washed off by a weeping nurse.”
That year proved especially distressing for Starzl. It was marked by four more liver transplants, and four more deaths, with each patient surviving less than a month before succumbing to immune rejection or infection. Similar failures in Boston and Paris dampened the entire field for the next five years.

Like Carrel, Starzl continued to practice on animals. In 1964, Starzl produced what would become the first lifetime survivor after a liver transplant — a dog that lived twelve years before dying in old age. The key was azathioprine, an early immunosuppressant that became common for non-twin kidney transplants just two years earlier. Starzl treated the dog with azathioprine for 120 days and noticed that, unlike in kidney transplants, he could stop the immunosuppressive drugs after that time, and the liver graft would still survive. This observation, soon confirmed in pig experiments, revealed that the liver is “immunologically privileged,” largely avoiding the host’s immune attack compared to other organs. Maybe, thought Starzl, liver transplants were possible after all.
Emboldened, Starzl returned to human patients in 1967. He and his team transplanted a liver into a 19-month-old with liver cancer, named Julie, who survived for a whole year. Although her liver cancer ultimately returned, the success of the procedure itself proved that this operation was feasible. By the mid-1970s, a triple-immunosuppressive cocktail of azathioprine, prednisone, and antilymphocyte globulin pushed the one-year survival rate to 33 percent. Still, infections were so severe from the immunosuppression that death post-surgery was a common outcome.
A breakthrough occurred in 1979 with Starzl leading clinical trials using the newest immunosuppressant on the market, cyclosporine, in combination with steroids. This moment also saw a fundamental shift in protocol. Instead of waiting for rejection, Starzl began giving prophylactic immunosuppression to prevent the immune attacks. By 1980, the one-year survival had jumped up to 70 percent.11

By the 1990s, tacrolimus12 (Prograf) began to replace cyclosporine. Prograf worked by a similar mechanism of action — binding to a protein that helps block T cells from launching a full-scale immune attack — but was a hundred times more potent. In 2025, it is still the most prescribed immunosuppressant for liver transplants.
Such developments have made liver transplants not only a viable intervention but also a fairly successful one. Over 75 percent of liver recipients survive five or more years after surgery, and the longest living recipient of a liver transplant has had her liver for forty years.
The Allocation
Despite these advancements, livers still get rejected by transplant recipients 15–25 percent of the time, and doctors have no idea why. Rejections are often caught late because liver damage takes time to appear. Diseases return. Some people need a new liver two, three, or even four times. There can be clots, leaks, damaging scars, fatal infections, rejection episodes fought off with harsh steroids, and cancers brought about by the rejection medications themselves.13
It is partly because of these challenges that liver allocation is so difficult. Given the various complications that can emerge despite a surgeon’s best efforts, hospitals and medical personnel do everything possible to ensure that the organ will actually make it into the body of someone whose need justifies the risks.
While planning to watch Dr. Gibbs’s liver transplant surgery, I had a chance to sit in on an allocation meeting, which prioritizes potential recipients. Individuals who are approved are entered into the national database and put on the national waitlist. A hepatologist later explained to me that the liver list isn’t exactly a line as it is for kidneys, where potential recipients are assigned a position and wait until their turn comes. Instead, the list is dynamic, and the sicker one is compared to everyone else, the higher priority they become. Unlike kidney dialysis, no stopgap treatment can help when the liver fails — without it, one dies.
To determine who goes on the list, the team must first measure how sick the patient actually is. In Dr. Gibbs’s allocation meeting, liver specialists first introduce patients’ official liver diagnoses, followed by a number called the MELD score (Model for End-Stage Liver Disease), based only on a simple mathematical formula that considers blood levels of bilirubin, creatinine, sodium, albumin and a blood-clotting protein value. The MELD score runs from 6 to 40, and the higher your score, the more likely you are to die without a transplant.
There are obvious limitations to the MELD score. It doesn’t take into account your quality of life, ability to work, or any predictor of recovery. Sometimes, people with high MELD are more high-functioning than people with low MELD. And if your MELD is too low or too high, you probably won’t make the list at all. Sometimes, someone might move up and down based on “exception points,” such as whether they have lots of liver cysts, hepatic encephalopathy, certain cardiac failure risks, or certain tumors.
Establishing a patient’s status requires the transplant team to run a series of evaluations that often take two full days. They check heart function and dental health, run MRIs and blood tests, digging deep into medical histories to uncover potential complications. The allocation meeting then proceeds, with each specialist voicing concerns.
Anesthesiologists, for example, might describe a heart condition and an allergy to a certain sedative. Social workers might discuss whether the patient is in substance use counseling or whether they are mentally fit for such an intensive procedure.14 Financial specialists might ask whether a patient has insurance, as cost can frequently be prohibitive. Kidney transplants operate through a federal program that provides grants, but this is not true for livers, which cost about twice as much (around $446,000 for a kidney, and $1 million for a liver).15
The woman who would be receiving John’s liver had alcohol-associated liver disease, or ALD. Her MELD score fell mid-range. ALD is the number one indication for adult liver transplants. In response to this, most transplant centers in the U.S. require a six-month sobriety period for eligibility. Early in the history of liver transplantation, it was considered ethically and medically inappropriate to allocate the organs to people who had ALD. When transplants became more possible in the 1980s, the thinking was that six months of sobriety would either allow the sick person’s liver to heal enough to defer transplant or ensure that they would be more likely to survive the transplant by having demonstrated their ability to avoid alcoholic relapse.
However, such waiting periods are not supported by strong evidence. The problem then and now is that society can’t really decide whether alcoholism is a disease or a moral failure. It is often treated, confusingly, as both. The question of whether the six-month sobriety period is actually helpful remains unresolved.
A 2011 study was the first to seriously challenge the six-month sobriety rule by focusing on patients with alcoholic hepatitis, a sudden and severe liver inflammation that can kill within weeks. If these patients were forced to wait six months for a transplant, they’d die. So researchers transplanted them “early,” within a median of thirteen days from listing. The results proved striking: about 75 percent of early transplant patients survived the first six months, compared to just 23 percent of those forced to go through the traditional period. Among twenty-six patients who received early transplants, only three experienced alcoholic relapse.
A 2021 study expanded these findings to alcoholic liver disease more broadly, confirming that the sobriety period doesn’t predict who will relapse after transplant. Whether patients were sober for six months or not, their long-term outcomes remained roughly the same. The six-month sobriety period is now being challenged across the U.S. Even so, there are always more people on the waitlist than there are livers.
Ultimately, allocation has had two basic models: the first being the “designated-service” area, which is just geographical. The closer you are to the donor, the more likely you are to get the organ. This creates inherent disparity. It means that if you live in a region where there are few donors or lots of people, you’re more likely to die while still on the waitlist. Someone who is moderately ill in, say, Colorado (with one of the highest donation and transplant rates) will get an organ before someone who is acutely ill in, say, California (with one of the lowest rates).16
In 2018, six waitlisted liver transplant patients in New York, California, and Massachusetts filed a lawsuit challenging this geographic bias. The legal challenge forced a nationwide policy shift. In 2020, the Organ Procurement and Transplantation Network (OPTN) adopted the second basic model, the “acuity circle model” for liver allocation — a system that prioritizes how sick you are rather than where you happen to live or which hospital system you’re part of.
Such a change might sound beneficial, but if you ask Dr. Gibbs about it, he’ll tell you that it also means surgeons’ having to get on more planes and travel longer distances for organs, the undermining of local donation circuits, the increasing of the financial burden on hospitals, and, of course, keeping organs on ice for longer periods of time, which risks greater degradation.
These are just a few of a bevy of tradeoffs involved in the ethics of transplants, however. Some questions surrounding the procurement of organs have existed from the very beginning, whereas others, such as whether an equitable and non-exploitative market for organs can exist, are much newer.
Bioethicist Dr. Arthur Caplan, who heads the medical ethics program at NYU, considers what it would mean to have more donors. “One way to look at it,” he told me, “is that any improvements in public health measures, better airbags, better car seats, better cardiovascular health, no alcohol, no fentanyl, reduce the number of donors.” He’s referring to the most common reasons that donors end up brain-dead at a hospital. In the state where I observed the transplant, for example, the leading cause of death in 2022 for adults under the age of forty-four is fentanyl overdose. (A few weeks after witnessing this transplant, I’d go to the local procurement clinic, where I’d encounter a brain-dead donor my age who had fatally overdosed on fentanyl in his car.17) Dr. Gibbs echoed my ambivalence when he conceded that these deaths represent “unbelievable tragedies and unbelievable miracles.”
Complicating Dr. Gibbs’s assertion are conversations around other means of organ donation — namely, the creation of an organ market.
The U.S. still largely clings to altruism as the prime motivation for organ donation. But with people dying on waiting lists, economists keep pushing for market-based incentives that could change the dynamics of donation. Take kidneys, which, unlike hearts or lungs, can be donated by living people. Why shouldn’t there be a market for these, as the one remaining to a donor is sufficient to maintain normal bodily function? A market for livers would be more challenging because a full liver cannot be removed from a living donor — only partial lobe donations are possible, and those carry high surgical risks. But still, selling a portion of one’s liver lies within the realm of possibility.
Iran already has an organ market. There, you can sell your kidney for any price you negotiate, and there’s no waiting list. A kidney costs about $4,200, expensive but within reach, especially with charities helping to cover costs. Though unsettling to many, this system has its merits. With a greater supply and lower prices, more lives could be saved.
Understandably, many countries are reluctant to permit such direct commodification of body parts. So economists have proposed subtler options. The Netherlands offers generous compensation to kidney donors — covering everything from transportation to dog-walking during recovery, plus three months of paid leave. Perhaps as a result of such incentives, they have one of the world’s highest live donation rates. Meanwhile, Israel takes a different approach, giving organ donors and their families priority access to transplants if they, themselves, ever need one. Even something as simple as checking the organ donor box on your driver’s license could come with reciprocal benefits — knowing that your willingness to give might translate into priority if you ever need to receive. Depending on one’s point of view, these incentives to donation could seem like progress or coercion.
Finally, there is the tradeoff between surgery and prevention. What makes the liver different from all other organs is that many of its foremost diseases are preventable. In women, the most common indication for liver transplant is MASLD,18 a metabolic disorder characterized by increased fat in the liver, and associated with diabetes and obesity. It’s estimated that about 38 percent of adults worldwide have it, and most of us don’t even know it. While this number is alarming, the actual fraction of cases that will lead to liver failure is relatively low. The trouble seems to be more like a background noise in the body that can exacerbate troubles beyond the liver itself — an increased risk of heart diseases, cancers, and overall mortality.
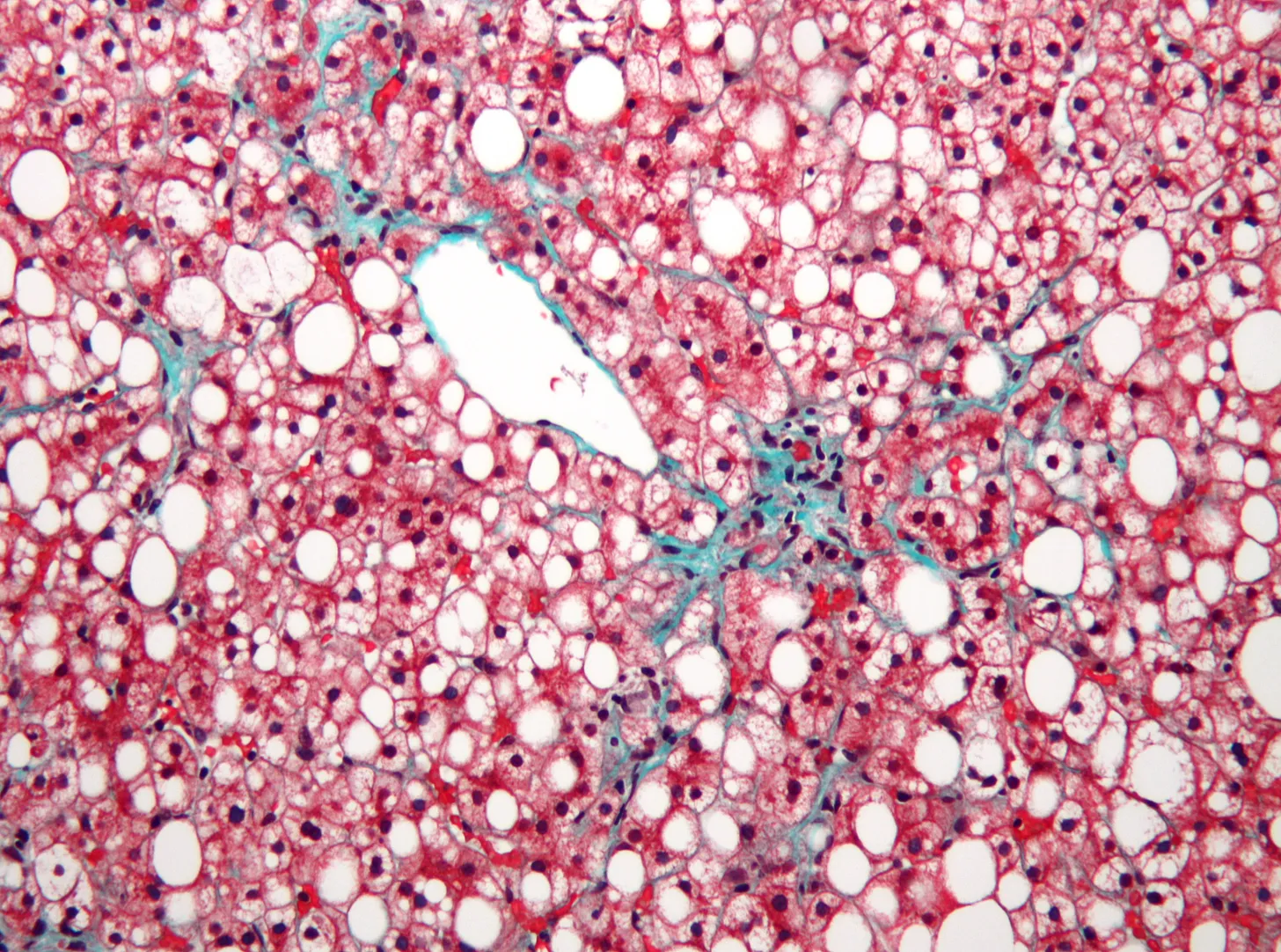
Both MASLD and alcoholic liver disease are (to a point) preventable and reversible with the usual repertoire of familiar public health recommendations (e.g., eating more vegetables, exercising more, and abstaining from alcohol). The problem, however, is that prevention is tedious and boring, and habit change is hard. Parallels can be found in the recent rise of GLP-1 receptor agonists like Ozempic to combat obesity. The popularity and stunning efficacy of these drugs have helped us admit that prescriptions are easier than lifestyle changes. Beyond illustrating the power of therapeutic shortcuts, however, these digestion-slowing drugs, originally for diabetes and weight management, have recently been shown to also reduce the fat in the liver. If the early research about these same agonists helping with alcohol addiction turns out to have legs, then GLP-1s could potentially eliminate the top two indications for liver transplant.
Even as we acknowledge the many logistical and ethical challenges that surround liver transplantation, we must not lose sight of the astonishing fact that it is even possible. John’s liver, rushed across state lines on its bed of ice, was about to give a stranger a second life.
The Recipient
In the operating room, Dorothy lay draped and waiting. Chief surgeon Dr. Gibbs was in command. He is a short man with rosy cheeks and over three decades of experience. Every ten minutes, his cell phone would ring, and a scrub nurse would answer and relay logistics for yet other livers and kidneys.
The major difference between the transplant operation and the procurement was that Dorothy was alive and would, if all went well, wake up. Dr. Gibbs radiated easy concentration as did the surgical fellow across from him. So far, Dorothy was not a difficult case. She wasn’t bleeding out, and she showed no signs of complications. They had already sifted through her diseased liver’s attachments, cutting through its embryonic planes with the bovie and sealing up leaks. Now, they were tying off its circulation in order to remove it.
Meanwhile, John’s liver was being prepared for transplant. It sat in a bucket on the table, where a surgical assistant checked the floppy vessels for holes. This was normal — as a liver is removed, the tiny vessels branching off larger ones tend to fall away and leave little openings. The surgical assistant squirted liquid through the latter and, upon finding a leak, would stitch it up.
Dorothy was swarmed with gloved hands. The surgeons lifted her liver out and plopped it into a bucket. It clearly looked diseased — engorged, lumpy, and mottled. Without her liver, the cavity of her body looked completely empty, just like John’s had. The two livers rested side-by-side in their respective tubs, John’s bloodless and gray, hers, misshapen and hard, and I thought how strange this exchange of life was.
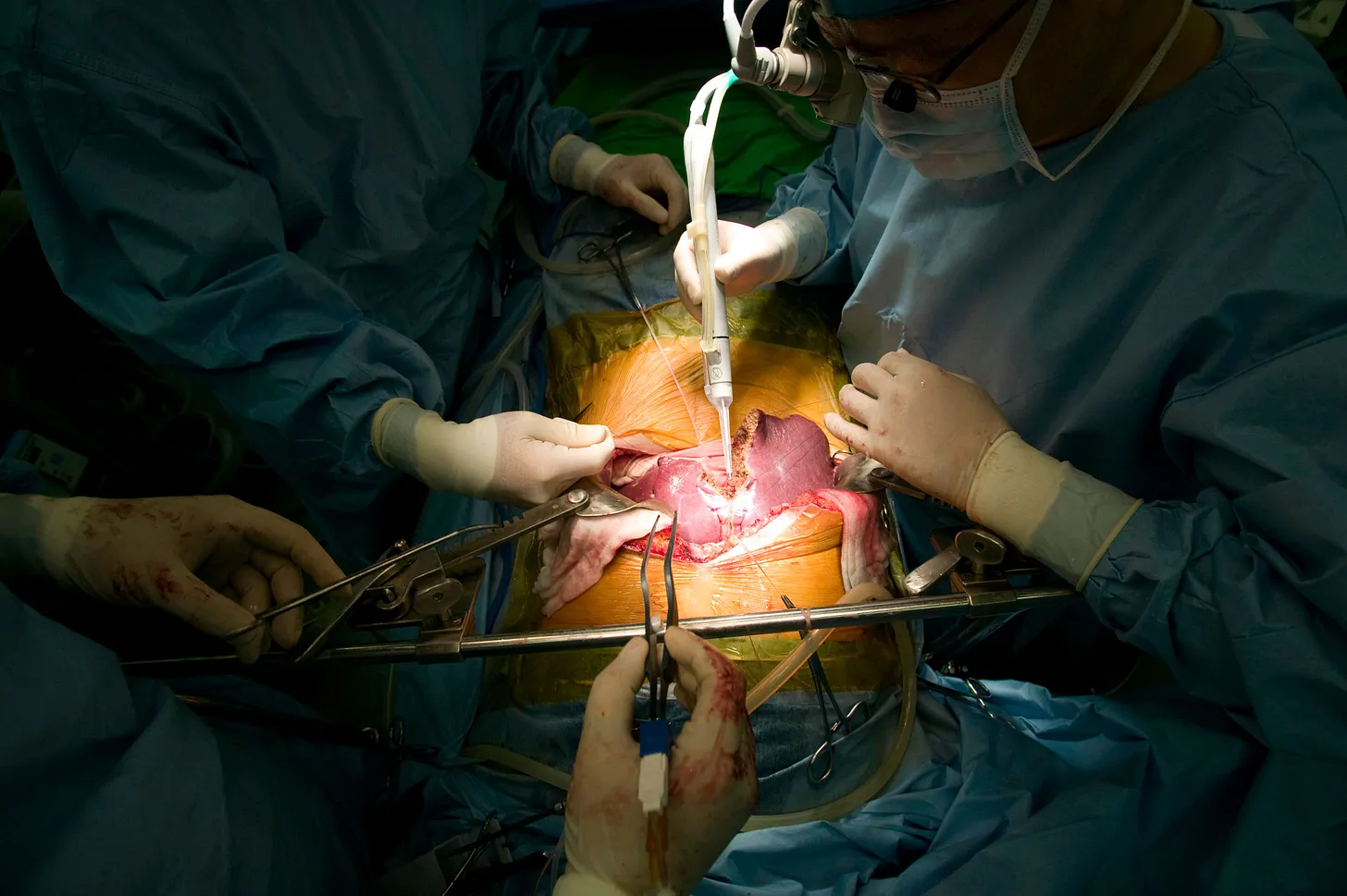
Everything began to move quickly. Dorothy’s circulation was at stake, and the anesthesiologists at her head monitored her carefully. Despite the bustle, Dr. Gibbs stayed calm. Holding the new liver over her body, he and his fellows lowered it into the abdomen, its wiggly vessels looking like white, bloodless noodles.
Deftly, they attached the vessels in the opposite order in which they had been cut from John. First, they reattached the vein that drains out from the liver and brings blood back to the heart — the one that had gushed blood into John’s cavity in its final circulation. Second, they connected the portal vein, uniting the liver to the intestinal circulation, using a permanent suture called the baseball stitch. Next, they attached the hepatic artery — the one that carries blood from the aorta to the liver. This technique is still called Carrel’s patch. Then, the bile duct was connected while the fellows dabbed the inside of the abdomen with towels, keeping it dry. Finally, her wound was stapled closed.
Dorothy’s surgery was over, and her healing could now begin. Dr. Gibbs’s team removed all the sheets, and I saw her naked body for the first time, her clean toes, her mildly yellow skin, her chest splotchy with little red starlets, where tiny blood vessels had leaked beneath the surface, a telltale sign of her formerly failing liver. The doctors started to remove the tubes snaking into Dorothy’s body. Her face was slightly puffy, but otherwise, she looked as if she could have been sleeping. The anesthesiologist began to reduce her ethers, bringing her back to earth little by little, tapping her forehead, saying her name, snaking a long tube out of her mouth, and stuffing a wad of cotton between her teeth to catch the bleeding.
Suddenly, she lurched and inhaled, eyes opened wide, like a newborn or someone suddenly resurrected. She groaned. The nurses held her tenderly, like they’ve probably done thousands of times before: You’re okay. You’re just waking up, they told her. You’re on a very narrow cot. Relax, we’ve got you.
With a concerted effort, they moved her to a recovery bed. Blue eyes open and empty, she stared at the ceiling. She didn’t register us, and I wondered where she’d been during her surgery and if she’d be a different person when fully conscious once again. A transplant psychologist told me that having a liver transplant is like getting a personality transplant. Perhaps the changes come from the relief of no longer having to wait for an organ or suffer pain, or perhaps, they’re due to something more.
“The biggest difference I notice is laughter,” she said.
Dorothy would be placed on a strict regimen of immunosuppressants that would allow her body to tolerate the alien organ instead of destroying it. These pills would keep her alive. Additionally, she would get a month or so of steroids to keep inflammation at bay, as well as an assortment of antifungals, antibiotics, and anti-anything that could get into her wound and take advantage of her vulnerable state.
By mid-morning, a fellow walked me over to Dr. Lee’s office. I changed back into my street clothes. Dr. Lee asked me if I wanted to join her on the flight to Michigan for another liver. At this point, I’d been awake for twenty-seven hours and could only imagine how long Dr. Lee had been awake. I said no thanks. Dr. Lee didn’t take it amiss.
“I should probably have lunch,” Dr. Lee said. But without further ado, she climbed into a blue-striped ambulance once more, red ear warmers and all, to be whisked away. I sat on the stoop and waited for my boyfriend to pick me up. While people in scrubs milled about and ambulances circled, I scarfed down an entire bar of chocolate and thought about how Dr. Lee would probably forget to eat.
Dorothy’s diseased liver wasn’t yet at the end of its own journey. A trained staff member carried it in a big bucket from the operating room to the pathology lab. There, it would be “fixed” in formalin, making it rubbery, easy to slice, and safer to manipulate without transmitting viruses. The pathologists would take little sections of it, covering each with special wax, shaving it, and staining it pink and purple to highlight its terrain.
Eventually, the in-house liver pathologist will look at Dorothy’s samples through her microscope. She will, as she puts it, “appreciate what disease this liver has to show.”
The other prepared sections might be kept in the hospital for decades or transferred to a medical storage facility nearby, where they will stay until needed or discarded. The remainder of the organ itself, still in the white bucket, will be brought to an incinerator by a trained waste manager, where it will be burned into ash.
In the end, this surgical journey is just one story of many that can be told about this remarkable organ. In 2,000 BCE, Babylonian kings employed augers to read the future in slaughtered sheep livers. Between 1,000 and 30 BCE, the Etruscans forged bronze livers etched with the names of gods. Plato wrote of the dreams projected onto the surfaces of livers in his Timaeus in 360 BCE. While some of these tales are mythic, others, just like transplant surgery itself, are edging their way into reality.
When Zeus needed to punish fire-stealing Prometheus, he chose the Titan’s liver as the site of his eternal torment, making it regenerate each night only to be devoured again at dawn. The liver’s weight, rich blood supply, and obvious importance made it a logical subject for Greek mythmakers. But of course, ancient writers didn’t realize that the liver actually is the only organ that can fully regrow to the exact size the body needs to function properly, even if 75 percent of it has been removed. It seems fitting that the divine fire of insight they envisioned Prometheus giving humanity now allows us to study the regenerative powers of this organ.
These regenerative properties are driving much of today’s research into liver function. In 2025, researchers in Spain discovered how it begins repairing itself just minutes after injury. It turns out that the liver fires off a molecular signal, called glutamate, to the bone marrow’s immune cells, coaxing them to repair damage. We could imagine glutamate as a supplement, a stopgap to keep patients alive while they wait for or recover from a transplant. Other scientists have pinpointed the switches that put the liver’s daily work on pause, rerouting energy into rebuilding its damaged tissue. And today, clinical trials are probing whether macrophage and stem cell therapies can push even a liver as damaged as Dorothy’s back to a healthy state.
In a world where liver health reflects the vices of society but also its triumphs, this ought to give us hope. Replacement may have given us miracles, but regeneration might prove the body is its own best donor.
{{signup}}
{{divider}}
Donna Vatnick writes essays that explore scientific discovery and its most passionate devotees. Before completing her MFA in nonfiction, she worked in molecular biology labs and coordinated clinical trials in Boston. Her writing can be found at The Los Angeles Review and The Millions.
Cite: Vatnick, Donna. “A Liver on Ice.” Asimov Press (2025). https://doi.org/10.62211/29wp-55ye
To protect patient privacy and the identities of medical professionals, all names in this story are pseudonyms and locations have been generalized. Certain details have been obscured for the sake of confidentiality. I am deeply grateful to the village of surgeons, hepatologists, researchers, transplant donors and recipients, editors, fact-checkers, and friends who made this story possible. Lead image by Ella Watkins-Dulaney.
Footnotes
- All names have been changed at the request of the surgeons and hospitals involved in this story.
- If the power of attorney doesn’t answer, other relatives are asked in order of their significance: spouses come first, adult children come before parents, then siblings, adult grandchildren, grandparents, and finally, close friends and the estate guardian.
- This includes screening for infectious diseases, obvious cancers, past medical history, past risky behaviors, or anything else that could cause complications for the recipient.
- The longest surviving brain-dead person was only four years old in 1984 when he got bacterial meningitis and lost brain function. Spending the next twenty years unconscious and on a ventilator, the boy hit puberty, had an active immune system, and regulated his own blood pressure while his brain slowly “mummified” into a hard crust. He died biologically at twenty-four of a heart attack. Does that count as living? In such cases, does the ventilator provide “life-support” or merely delay death?
- You can find this account in the Sushruta Samhita, one of the oldest known surgical texts in the world. It describes “cool-headed physician” using a broad “leaf of a creeper” as a template to size the graft, swiftly slicing a patch of living flesh from the cheek, attaching it to the severed nose, bandaging the wound, inserting two small tubes into the nostrils to maintain breathing, and applying pulverized herbs and oils until healing is complete.
- According to Craddock in Spare Parts, Denis’ first transfusion attempt probably took much longer than later ones, so it’s possible that platelets naturally clogged the tubes, preventing any animal blood from actually entering the patient in this particular case. Most likely, the boy would have recovered even without the attempted transfusion.
- The appeal of animals, for some experimenters, was the Cartesian idea that animals were machines and, therefore, felt nothing — and that the alternative, taking tissue from humans, would be immoral.
- Apparently frog skin was a preferred choice for skin grafts because they could be found in abundance in the countryside where the doctor lived (except in the winter.)
- Carrel was so obsessed with precision that his laboratory — walls, floors, ceiling, tables, cabinets, surgical gowns — was fully black, and a skylight shone directly above his operating table. He wanted to cut out all possibility of glare and see every speck of dust. Unfortunately, he employed this same fastidiousness authoring books on eugenics and the decline of the human race.
- Craddock (2022), p. 221.
- The advances of the ‘80s meant that the market and infrastructure had to evolve in step with these transplants. Better preservation solutions doubled the time organs could survive on ice.
- Cyclosporin and tacrolimus were both discovered thanks to soil samples. Cyclosporin came from a white mold fungus found in Norwegian dirt, while tacrolimus was uncovered in the earth near Japan’s Mount Tsukuba.
- The mental weight of this procedure is also a complication. Even the most prestigious hospitals lack resources for transplant-specific support. One transplant recipient (operated on by Dr. Gibbs) told me that if she had a million dollars, she’d make sure every transplant patient could be paired with a transplant psychologist.
- A major point of conversation is whether or not the patient has a support system. To receive a transplant, a patient is required to have a designated caretaker because, without one, they probably won’t survive.
- Officially, this disparity exists because when the nation-wide transplant matching system was established in 1984, kidney transplants were medically accepted, while liver transplants were still considered largely experimental.
- Steve Jobs, who needed a liver in 2009, put himself on the waitlist in Memphis, Tennessee, instead of his actual residence in California because in California, the wait times were so much longer, and he would have to be much sicker (with a higher MELD) to be eligible. This is called “dual listing.” His transplant was a result of what is called “transplant tourism,” where, if you’re wealthy enough, you can go “live” somewhere where the lists are shorter and you don’t have to wait to get sicker. Not only is the geographical system inequitable, but it can also be exploited.
- The fentanyl poses a more immediate danger to respiration rather than liver damage, so these victims are often suitable candidates for organ donation.
- A move is currently underway to change the name of nonalcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated steatotic liver disease (MAFLD).
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.