Pausing Insect Activity

For all known life forms, activity is punctuated by periods of rest. Sleep may be the most familiar, but many other distinct dormant states occur on longer timescales, from weeks to months on end. Such suspended animation has garnered many different names: hibernation and torpor (in mammals and birds), brumation (in reptiles), diapause and quiescence (in insects and nematodes), aestivation (summer dormancy in vertebrates and invertebrates), hypobiosis, cryptobiosis, and latent life (in microorganisms).
This abundance of terms is a consequence of the historically siloed nature of dormancy research — the phenomenon has been noted and described independently in different organisms across centuries. The most well-known of these, however, is likely hibernation. Not only is it an endearing behavioral trait belonging to charismatic fauna like bears and groundhogs, but it is also relatively easy to observe in nature. Where a brumating lizard might not attract much notice, a disheveled bear stumbling out of a den certainly does. And even if it seems far-fetched, the mechanisms behind mammalian hibernation are of increasing scientific interest due to their potential in human life extension and space travel.
While hibernation commands the most attention, a growing body of research suggests that all forms of dormancy reflect similar underlying physiological, metabolic, and gene regulatory mechanisms. Indeed, the dormancy observed in non-mammalian species is no less gripping and ecologically consequential than mammalian hibernation.
In no other group of organisms does the sheer scale of programmed dormancy stand out as staggeringly as in insect diapause. Unlike most other kinds of dormancy, largely characterized by physiological and behavioral suppression, insect diapause is a state of programmed developmental arrest.1 It’s a pause button pressed on one of the stages of their life cycle — either as eggs, larvae, pupae, or adults, depending on the species.
As such, diapause removes a tremendous portion of insect biomass from active life for large chunks of each year — up to 9 months in some species.2 At any time, there are an estimated 10 quintillion (1019) individual insects alive, collectively weighing about 1 billion metric tons. Depending on the season and locale, a significant percentage of this biomass can be locked in a dormant state — higher in temperate latitudes, lower in the tropics.
It follows that knowledge of the timing and mechanisms of diapause has massive economic and ecological consequences in agriculture, forestry, vector-borne disease spread, and more.
{{signup}}
Diapause Hijacks Developmental Checkpoints
The capacity for dormancy has emerged multiple times throughout biological history. Ancestral microscopic life forms in the ocean experienced boom-and-bust cycles in nutrient availability and other planetary cataclysms. In response, like the Trisolarans from Cixin Liu’s The Three-Body Problem, they developed reversible suppression of metabolism as a bet-hedging strategy to survive Earth’s own “Chaotic Eras.”
When life forms emerged from the oceans and started colonizing the land, they had to reckon with seasonality that is much more starkly pronounced in terrestrial than in marine habitats. This was not merely reactive — multiple taxa like plants, insects, molluscs, and vertebrates harnessed the predictability of seasonal changes to develop an anticipatory response, using photoperiod (duration of daylight), temperature, and other cues as an advanced warning.3 These dormant states served as “time capsules,” buffering them against complete population collapse in a changing environment.
Dormancy in insects was seen as analogous to mammalian hibernation and was described well before the term “diapause” was introduced into entomologists’ vocabulary. As early as 1734, the French naturalist René Antoine Ferchault de Réaumur published a study on bee physiology and behavior in winter.4 He wrote:
I know of no insects to which heat is so necessary. They perish with cold, in an air temperature which appears good enough to all other insects of our climate. The cold … puts the bees into a state in which food ceases to be necessary to them; it holds them in a sort of numbness, during which they stop sweating or, at least, during which the quantity of what they sweat is not considerable that it may not be repaired by food, without their life running at risk. In winter, while it freezes, the hives without transparent walls can be considered without fear; for they can be laid on the side, or even turned upside down, without putting any bees in motion. They are seen piled up and pressed very closely together … If thaw arrives, if the temperature softens, and especially if sunrays hit the hive and warm it up, the honey-flies emerge from their kind of lethargy; they wave their wings, they set in motion, and activity is restored to them.5
But the term “diapause” was coined in 1893 by the distinguished American entomologist William Morton Wheeler to describe the state of locust and grasshopper eggs during winter.6 He observed that during their embryonic development, the embryo undergoes circular movements within the egg, but that there’s a period of rest between these movements where no development is noticeable — what he referred to as a diapause.
Some insects incorporate diapause into their development as an obligate, unskippable stage, while others use environmental cues like day length, temperature and humidity to determine whether or not to enter diapause and delay development until favorable conditions are present. In that sense, diapause is an example of phenotypic plasticity, the capacity of one set of genetic instructions, “genotype,” to produce two or more alternative phenotypes (sets of traits), depending on the environment. In the case of diapause, these alternate phenotypes relate to distinct trajectories in the insect life cycle.
The seasonal diapause program is initiated long before adverse environmental conditions appear. In preparation for diapause entry, a number of biochemical and cellular changes take place. Cells stop dividing, and fat bodies of insects (equivalent to adipose tissue and liver in mammals) accumulate large fat reserves and storage proteins as an energy source during the long period of fasting in winter. Metabolic rates decrease, and aerobic metabolism (oxygen-dependent) switches to the more conservative anaerobic (oxygen-independent) form.7 Insect bodies also become more stress-resistant, thanks to fortification of their cuticle and expression of protective molecules like heat-shock proteins and antioxidant enzymes.
Insect development is uniquely modular in that successive developmental stages are demarcated by sharp transitions like molts and pupation. Holometabolous insects like butterflies, beetles, flies, and ants undergo complete metamorphosis, consisting of four stages: eggs, larvae, pupae, and adults, with the larval stages (called instars, separated by molts) that look very different from the adult. Hemimetabolous insects like grasshoppers, dragonflies, cicadas, and termites have larvae called nymphs or naiads (in aquatic species) that resemble adults in miniature and don’t pupate.
The modular nature of insect development, with programmed stops and starts, lends itself to the entry into diapause at any of the stage transition checkpoints. And indeed, diapause has been observed at all of these checkpoints across various insect species, from eggs to adults. Even so, diapause typically falls before differentiation at the beginning of a developmental stage, or after its completion, but not halfway through. An exception to this is embryonic diapause, which can happen at any point during embryonic development, depending on the species. For example, spongy moths diapause as fully developed larvae encased within the egg, while migratory locust eggs arrest as early embryos, as Wheeler observed in his insect embryology experiments.
In order to arrest development, diapause relies on hormonal regulation. Hormones are, after all, the signals that determine how development proceeds at the molecular and cellular level. Stage transitions in insects are governed by two major hormones: ecdysone and juvenile hormone. Ecdysone is synthesized in pulses (its levels sharply rise, reach a peak, and fall) before each molt, while the juvenile hormone levels are graded throughout development. Molting from one larval stage to another happens when ecdysone is combined with high levels of juvenile hormone. When juvenile hormone levels are low in the late larval stage, ecdysone prompts pupation and metamorphosis.
While diapause can also happen at the pupal stage, it is not the same as metamorphosis: in fact, it delays metamorphosis if it coincides with the beginning of pupation. Metamorphosis involves massive restructuring of pupal tissues into adult ones, and while it externally also may seem like a period of rest, it is a very active process from within. Diapause, in contrast, can happen at any developmental stage and puts insects into a state of developmental arrest and low physiological activity, temporarily interrupting advancement to their next stage.
Insect hormones, much like our own, are not just governed by internal signals, either. Environmental cues like temperature, photoperiod, and nutrition also influence the hormonal system which, in turn, dictates development. In temperate latitudes, this usually means late-summer cues like shortening daylengths, cooling temperatures, and, for many herbivores, the declining nutritional quality of aging host plants compared to fresher spring sources.
In addition to these natural cues, it is also possible to induce or prematurely terminate diapause in various species using an array of natural and synthetic chemicals. The range of these substances is truly staggering — among them are weak acids and bases, organic solvents (like dimethyl sulfoxide, DMSO), toxins, heavy metals, LSD (lysergic acid diethylamide), carbon dioxide, deuterium oxide, and hydrogen peroxide. Most peculiarly, each of these substances can affect diapause only in a handful of insect species — there’s no universal diapause inducer or breaker.8
The ability to induce or terminate diapause using chemicals has enormous implications — with their help, we can artificially manipulate when insects are active and when they are dormant. If we actively interfere with diapause by altering its natural timing, crop pests could be forced into activity during the wrong time of the year when there are no available food sources or when it’s too cold for them to survive. This could include blocking insects’ entry into diapause in the fall, prematurely breaking them out of an overwintering, or causing them to miss the correct window for diapause termination in the spring, effectively driving them to ecological suicide.
Diapause for Pest Control
With the emergence of agriculture in the Neolithic era about 12,000 years ago, farmers had to learn about the natural cycles of plants and animals to exploit them efficiently throughout the year. And from early on, these first agriculturalists had to contend with plant diseases and pests that plagued their fields. Crop rotation — alternating the type of crop grown on a piece of land from year to year — is an ancient strategy for preserving soil health to improve crop yields.9 It is also one of the first systematic methods of pest control known to man, thanks to its ability to disrupt pest life cycles (which usually include an overwintering diapausing stage) dependent on these host plants.
The Food and Agriculture Organization of the United Nations estimates that 20 to 40 percent of global crop production is lost to pests each year,10 with invasive insects costing around $40 billion in crop and forestry damages. In the U.S. alone, invasive insects (not counting domestic pests) are estimated to inflict on the order of $30 billion each year in agricultural and ecological damages. Some of the most pernicious pests, such as western and northern corn rootworms, cause $1 billion/year in crop losses, and the variability of their diapause makes them especially difficult to fight.
Corn rootworms spend most of the year, from September to late May, as diapausing eggs. Once they hatch, first-instar larvae make their way to corn roots and start feeding, maturing to voracious third instars that cause most of the damage.11 Their effect on the corn plants is like that of severe drought, preventing the damaged roots from taking up water and nutrients from the soil. After feasting on the corn roots, the pests spend about a week in the soil as pupae, and the emerging adults continue feeding, now on the above-ground parts of the plant: leaves, corn silks (female flowers), tassels (male flowers), and corn kernels. Adult females lay eggs in the cornfields in late August and September, completing the insects’ full life cycle.

In the U.S. Midwest, corn rootworms were first described in 1823 by Thomas Say, known as the father of American descriptive entomology. But it was only in the 1860s and 70s that entomologists started paying close attention to this emerging pest.12 Up to that point, corn had been successfully grown on the same fields for several consecutive years. Faced with the exploding populations of these rootworms, even though the concept of diapause was not yet known, farmers were observant enough to rotate (alternate) the vulnerable corn with other crops like soybean, wheat, rye, or oats that the picky pest could not feed on.13 Crop rotation succeeded in protecting corn from damage, as the eggs from the previous year would hatch in a field of an inedible crop and perish.
But a few decades later, in the late 1920s, John Bigger, an Illinois Natural Survey Scientist, started documenting the first systematic breakdown of the effectiveness of this common two-year crop rotation (although he noted that longer rotations were still effective).
An explanation for this shift arrived in 1965, thanks to Dr. Huey C. Chiang, an entomologist at the University of Minnesota, who observed that a small percentage of northern corn rootworm eggs were capable of staying in diapause for two winters or longer. The pests caught up with the alternating cycle of crop rotation, which strongly selected against the wild-type one-year life cycle, favoring those insects that were able to persist in diapause longer. Crop rotation inadvertently selected for exactly those corn rootworms with a two-year prolonged diapause.14
This is an example of a bet-hedging strategy in action. Such variation may protect the population as a whole from going completely extinct, since at least some individuals can escape the adversity due to their unusual phenotype. And while good for the pests, it is potentially disastrous for their target crops.
It would be mistaken, however, to declare the pests’ complete victory over farmers. Crop rotation to this day remains an effective way to counter corn rootworm infestations. Rotating fields out of corn at least one in three years or on a portion of the total crop area every year can still significantly reduce selection pressure and help with pest management.
Knowledge of the location of overwintering sites is also critical for planning pest control efforts. One of the most damaging rice pests is the rice yellow stem borer. In their diapausing stage, the larvae overwinter at the base of the rice stem. It was found that if farmers flood rice fields for several days, they can eradicate up to 90 percent of these larvae. But this strategy is not as effective in wiping out the diapausing larvae of another pernicious pest species, the rice striped stem borer. These larvae, despite being in a diapause state, are still mobile and can escape the flooding rice fields, leading to low overall mortality (~10 percent).
Pest control can also happen “for free,” thanks to the natural enemies of pests — nematodes, spiders, or other insects that parasitize or prey on them in the wild. For example, coccinellid ladybirds prey on aphids, some of the most destructive sap-sucking pests of a range of cultivated plants. Ladybirds are an example of biological control agents, which can be harnessed for fortifying pest management efforts, as a finer and more precise weapon than crude pesticides or mechanical traps. The earliest known deliberate use of insect predators was recorded in China around 300 AD: The Asian weaver ant, Oecophylla smaragdina, had been successfully used for centuries to control citrus pests such as the lychee giant stink bug, Tesseratoma papillossa.

Insect predation is arguably easier to notice in nature than the more subtle and cryptic parasitism, so it took longer to put the latter into practice. Réaumur, whom we met earlier, wrote in the second volume of his six-volume series “Mémoires pour servir á l’histoire des Insectes” (published in 1736) about parasitoid wasps that naturally control crop pest populations.15 Parasitoid insects are those whose larvae develop within or in close association with another insect host, consuming the host’s tissues and eventually bringing about its death — unlike true parasites that don’t normally kill their hosts but live at their expense for as long as possible. Adult forms of parasitoids are usually free-living, but they seek out and lay eggs in or on the host.16
But the practical use of insect parasitoids had to wait almost another hundred years after Réaumur’s publication. In 1826, German botanist Theodor Hartig proposed to collect and store parasitized cabbage butterfly caterpillars to harvest parasitoid wasps that emerged from them, for later release. The beginning of the modern era of biological control is associated with the British-born American entomologist Charles Valentine Riley, who in 1888 imported Vedalia ladybirds as well as cryptic parasitic flies Cryptochetum iceryae from Australia to protect California citrus orchards from the pernicious cottony-cushion scale, Icerya purchasi. This undertaking was crowned as a resounding victory over the pest, and biological control took off as an efficient, cheap, and scalable method of pest management.
Many parasitoids are known to incorporate diapause into their life cycles. For the purposes of biological control, manipulating diapause makes it possible to synchronize large parasitoid populations in the lab at scale and have them ready to be unleashed on the seasonally peaking pest populations. This includes both being able to break diapause on demand and induce it artificially when needed for long-term storage. Diapausing parasitoids indeed have a longer shelf life (months instead of days or weeks) and lower costs of maintenance compared to continuously bred biological control agents.
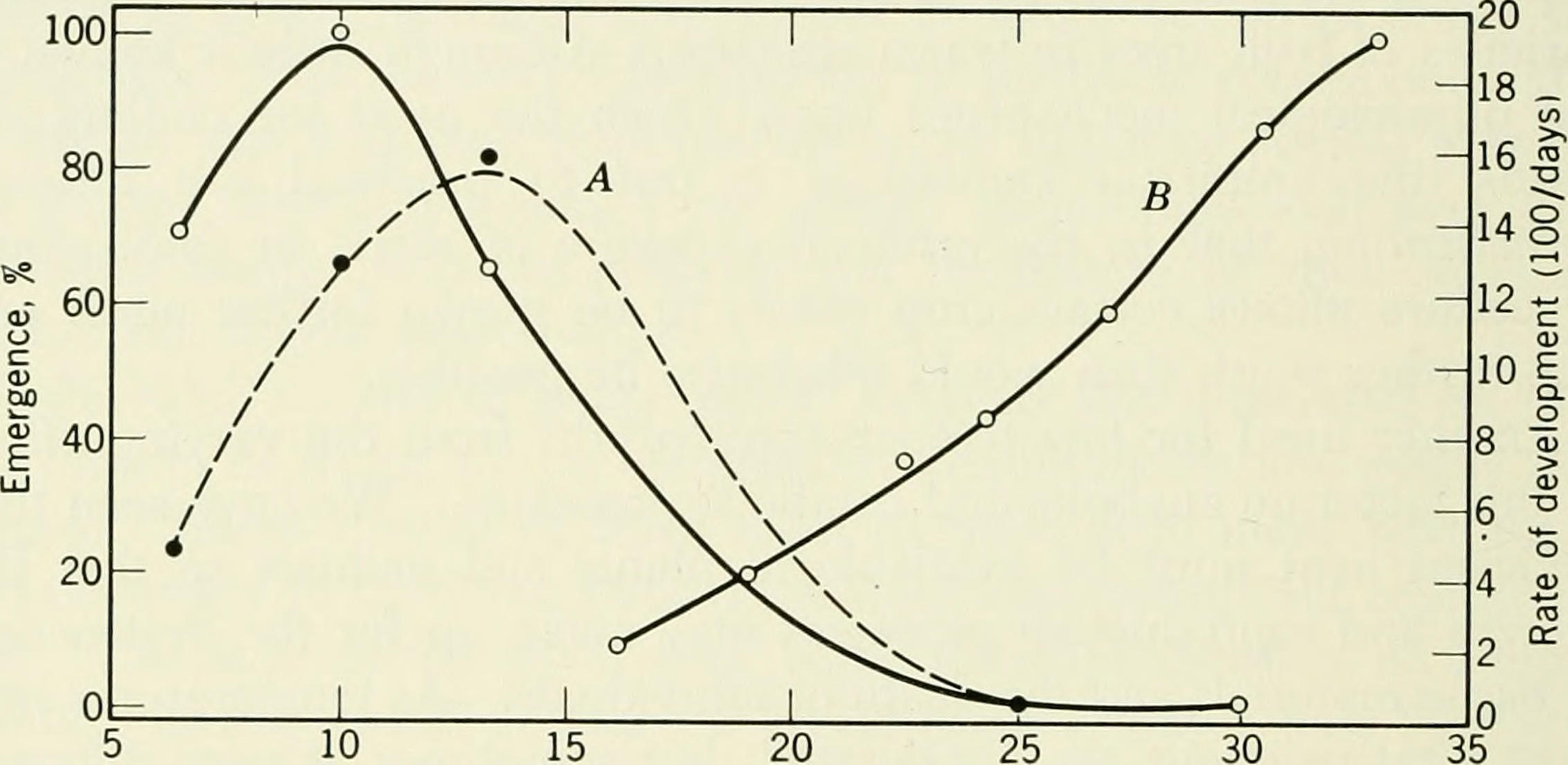
Still, diapause traits like the timing of its initiation and duration are very sensitive to environmental conditions and have to be carefully optimized for mass production. In China, for example, Trichogramma dendrolimi is used at an industrial scale against the Asian corn borer, which feeds on and destroys many valuable crops besides corn.17 T. dendrolimi can be induced into diapause through a two-step temperature regime (for example, 40 hours at 26°C followed by 31 days at 10 °C) or continuous exposure to low temperature. In laboratory conditions, T. dendrolimi is reared in the highly nutritious eggs of a substitute host, Chinese oak silkworm Antheraea pernyi, which is easy to breed at scale.

Trichogramma dendrolimi wasps can be kept in cold storage (at 1-3 ℃) in a non-diapausing state for up to three weeks, with their survival rate and fecundity worsening with increasing duration of storage. But while in diapause, T. dendrolimi prepupae can be stored for up to four months, and their survival rate, fecundity, growth rate, and efficiency of parasitism against the Asian corn borer in field studies are much higher compared to cold-stored, non-diapausing populations. Similar or even longer diapause-enabled shelf-life extension has also been found for other Trichogramma species like T. ostriniae (up to three months at 8 ℃) and T. maidis (up to 12 months at 3 ℃).
Diapause is not without tradeoffs, however. In some parasitoid species, it results in decreased fecundity, longevity, or flight activity post-diapause, to varying degrees. This diminished fitness, if it occurs, has to be weighed against the flexibility of storage and release timing that diapause affords. But based on the scale of active research on diapause induction currently happening in India, China, and the U.S., diapause use continues to expand in parasitoid rearing for biological control of a broad range of agricultural pests.
{{signup}}
Diapause in Insect Farming
Sometimes, of course, insects are not the bane of farming but the object of it. We farm insects — for silk, for honey, for animal feed — and understanding diapause in this context is also crucially important. Domestication of insects dates back to Neolithic China, around 4700 BC, when the mulberry silkmoth Bombyx mori was established as the foundation of sericulture — cultivation of silk.18 Diapause in Bombyx mori happens at the egg stage and is maternally programmed — its incidence depends on the temperature experienced by the mother, often regardless of the photoperiod.

Female silk moths reared above 21 ℃ produce a diapause hormone that acts on the ovaries to make them produce an unusual carbohydrate, sorbitol, in the egg yolk. Sorbitol induces developmental arrest and enhances cold hardiness of the embryos. Knowing this, Japanese scientists at the Institute for Silkworm Genetics and Breeding showed that diapause can be artificially induced in non-diapausing silkmoth eggs by soaking them in a sorbitol-containing culture medium, which enables long-term storage and transportation of eggs at any time of the year.
Conversely, in the early 20th century, French and Japanese zoologists discovered chemical interventions to artificially terminate or bypass diapause in silk moths on demand. Louis-Félix Henneguy in 1904 and Makita Kogure in 1933 published their studies showing that diapause can be terminated by brushing or immersing diapausing eggs in dilute hydrochloric or sulfuric acids, after a period of cold storage (30–90 days at 4 °C). This treatment is now a standard practice and makes it possible to continuously propagate silkworms for a year-round production of silk.
Another approach to interrupt silkmoth diapause is to use electric shock (corona discharge — a high-voltage direct-current treatment). These chemical and physical treatments would, in all likelihood, be lethal if applied to active non-diapausing insects, but diapausing eggs not only survive but also override arrested development in response to them.

Bumble bees were domesticated much later than silk moths, as late as the 20th century, with commercial rearing only starting in the late 1980s. Currently, there are over 2 million actively maintained bumble bee colonies worldwide. These bees serve as major pollinators of a variety of vegetables, fruits, and seed crops.19 In the wild, queens of bumble bees mate in the autumn and spend the winter in diapause. In the spring, they wake up and start new colonies.
A major part of bumble bee domestication involved attempts to bypass their long winter diapause, with an initial success coming from using manipulations of photoperiod and temperature.20 But in 1985, a German social insect physiologist Peter-Frank Röseler discovered a much simpler method: a short exposure of the queens to carbon dioxide (hypercapnia) prevents them from entering into winter diapause and stimulates oogenesis within days after treatment. CO2 narcosis in controlled atmosphere chambers is now routinely used for continuous rearing of bumble bees for greenhouse pollination.

Besides sericulture and pollination, insects are farmed for organic waste management and as feed for livestock or human food. Compared to other farmed animals, insects require significantly fewer resources, such as water and land, to produce, process, and distribute. Black soldier flies, Hermetia illucens, are a major and increasingly widely used species that feeds on a variety of decaying organic matter, like animal manure and vegetable waste. By converting organic waste back into live biomass that can be fed to animals, these flies efficiently close nutrition loops. Their larvae are rich in protein and fat and don’t retain hazardous chemicals like toxins and insecticides, thanks to a battery of specialized enzymes in their digestive system that efficiently degrade these substances.
An Israeli company called FreezeM has pioneered commercial deployment of suspended animation of black soldier flies with their PauseM® “neonate” (newly hatched) larvae, which can arrest development for 14-21 days with a 90 percent survival rate, allowing easier transportation and extended shelf life (they can also be kept in this state much longer, for 100 days or more, but at the expense of viability). PauseM® does not appear to be a true diapause, as there’s no extended preparatory phase, and larvae resume development immediately after being given access to abundant food. Instead, it is possibly quiescence induced by the rearing conditions. In any case, the logic of using this state of suspended animation is the same as in using diapause in other insect farming scenarios.21
But diapause can also be a nuisance when insect farming has to proceed continuously at a high pace. It can slow production rates simply because, with diapause included, the insect life cycles take longer to complete. Emergence rate from diapause is sensitive to rearing conditions, so there can be losses in viability compared to non-diapausing populations. Diapause is a double-edged sword that we can wield to our advantage or have to contend with and tweak, depending on our goals as insect farmers and researchers.

Diapause in Disease Vector Management
Beyond their benefits and damages to farming, some insects are also notorious carriers of disease and have plagued humanity for tens of thousands of years. A deeper knowledge of the diapause biology of these species might help against some of the worst human suffering brought on by insects.
By far the biggest disease burden and death toll among the insect vectors is inflicted by the Anopheles mosquitoes, carriers of malaria: WHO reported about 263 million cases and 597,000 deaths in 2023, most of them children under five. At least two Anopheles species, gambiae and coluzzi, undergo aestivation, an adult-stage summer diapause during the dry season (4-8 months), when the ponds in which the mosquito larvae normally develop dry out. During this time, they hide in cryptic sites and are hard to locate. But a few days after the first rain, the observed mosquito numbers surge over 10-fold.
A 2011 study from the Laboratory of Malaria and Vector Research at the National Institute of Allergy and Infectious Diseases (NIAID) showed that weekly indoor pyrethrum sprays (potent insecticide derived from dried flowers of the daisy Chrysanthemum cinerariifolium) throughout the dry season reduced the population of the aestivating Anopheles gambiaeby 30 percent at the onset of the rainy season. Despite the tremendous implications of aestivation, little is known about its mechanisms in Anopheles mosquitoes, and there are currently no scalable attempts to manipulate it in the wild. The ability to disrupt their diapause or time the sterile insect technique and mass insecticide treatments to their emergence from aestivation could prove promising in preventing malaria transmission.
Diapause is also a part of the life cycle of other disease vector mosquito species like the house mosquito, Culex pipiens(which transmits West Nile Virus, WNV), and the Asian tiger mosquito, Aedes albopictus (carrier of Dengue, chikungunya, yellow fever, and Zika viruses). In Culex pipiens, diapause happens in the adult stage (reproductive diapause), and its incidence in early fall coincides with the decline in human WNV cases. Diapausing females don’t feed on blood and are generally less active than non-diapausing ones. This points to a window of opportunity for applying control measures to mosquito populations prior to diapause induction, which would not only curb them in the following spring but also hinder WNV from overwintering, reducing the resulting disease burden.

The Asian tiger mosquito is native to Southeast Asia, but in the past 40 years, it has been spreading globally. As a carrier of at least 26 arboviruses and an aggressive biter, this mosquito is a major target of vector management programs. Unlike most Anopheles and Culex mosquitoes, Aedes predominantly overwinters as diapausing eggs (or rather, as fully developed larvae encased within eggs) that are laid by females in the autumn in response to shortening day lengths. Knowing this, larvicidal control can be applied in the early to mid-spring when the eggs exit diapause, hatch, and resume development (over about 7 weeks), and few adult mosquitoes are present.
Since the timing of intervention is so crucial in fighting disease-transmitting insects, knowledge of their diapause can help us move from reactive suppression of the already abundant and active vectors to a pre-emptive attrition when they are at their most vulnerable, as newly hatched and developing larvae or adults that have just exited a reproductive diapause.
A Delicate Instrument
The impact of diapause on the insect life cycles is staggering. The diapause process extends from the perception of environmental signals to initiating the preparatory pre-diapause stage, to diapause itself, and to post-diapause alterations of physiology and epigenetics. In some cases, it may even carry over across generations. Maternally programmed diapause exists in silkworms Bombyx mori, mosquitoes Aedes albopictus, the jewel wasp Nasonia vitripennis, and many other species.
In some species, the diapause programming effect can span several generations, not just immediate offspring. A grandmaternal temperature effect has been described in the egg parasitoid Trichogramma tenegai, while in Trichogramma principium, diapause experience in a female inhibits the ability to enter diapause in her offspring up to the 5th generation.
This remarkable evolutionary strategy doesn’t come without costs, however, both to the diapausing insects themselves and to their progeny. Experience of diapause may result in lower fecundity and smaller body size than in non-diapausing conspecifics. Metabolic suppression during diapause helps conserve energy resources, but as those resources are depleted, loss of weight and lipid stores ensues. This has been documented in many insect species, including the flesh fly Sarcophaga crassipalpis, the adzuki bean borer Ostrinia scapulalis, and the parasitoid Doryctobracon areolatus.
But there are examples to the contrary as well, especially in insects that undergo adult diapause, where diapausing adults are larger and more fecund than non-diapausing ones, as in the scorpionfly Panorpa vulgaris. Adults of the swallowtail butterfly Papilio xuthus are smaller if they emerge from diapausing pupae, and their offspring grow faster than the progeny produced by non-diapausing females.
Given the massive impact of diapause on insect life cycles, our ability to manipulate it amounts to a powerful intervention that touches on insect welfare in various contexts: public health, agriculture, forestry, and conservation. Disrupting natural diapause in pests and disease vectors may still be a better approach than killing them with pesticides that can also inflict collateral damage on other insects. In general, as we understand the mechanisms of development, physiology, and behavior of animals better, we can interact with them in ways that are intentional rather than crude and reckless.
O, it is excellent
To have a giant’s strength, but it is tyrannous
To use it like a giant. –
says Isabel in Measure for Measure. Knowledge of diapause indeed gives us “a giant’s strength,” by means of a more delicate and refined instrument with which to intervene in insect life. Used with discernment, it can help us avoid the unnecessary damage that would otherwise occur in less well-timed and species-specific interventions.
That said, while the basic underlying hormonal and physiological mechanisms of diapause are shared among insects, much of diapause biology is still unmapped. There are over a million known insect species, with a dizzying variety of life strategies and ecological niches that they occupy (not to mention that an estimated 80-90 percent of all existing insect species remain undiscovered).
Given the staggering effect of insect biomass on all of terrestrial life, deciphering diapause in each species can have outsized economic and ecological consequences. It not only influences what we plant and when, but also how we put our fingers on the scale of insect life — which species to subdue, and which to propagate. Ultimately, whether one is motivated by impact or purely the complexity and beauty of biology, the study of insect diapause stands apart, revealing new ways of interacting with the most abundant class of animals on Earth.
{{divider}}
Ulkar Aghayeva, a staff writer at Asimov Press, writes about biology, history and philosophy of science. Her science history blog is called Measure for Measure. Ulkar also writes about music history and cognition on The Bass Line.
Thanks to Dr. Jeffery Tomberlin at Texas A&M University for helpful discussions on insect diapause and especially the biology of the black soldier fly. Lead image by Ella Watkins-Dulaney.
Cite: Aghayeva, U. “Pausing Insect Activity.” Asimov Press (2025). https://doi.org/10.62211/72jr-22fg
Footnotes
- Some mammals too can arrest development as embryos, at the blastocyst stage. This is known as embryonic diapause, or delayed implantation, and it has been described in over a hundred eutherian and marsupial species, including rodents, minks, bears, and armadillos. In contrast, hibernation can occur at any age and involves massive physiological, metabolic, and behavioral changes, before, during, and post-dormancy.
- Some insects can spend several consecutive years in diapause. For example, the midge Contarinia tritici often overwinters for 3 years, the beetle Leptinotarsa decemlineata can be dormant for up to 9 years, and the Yucca moth Prodoxus y-inversus can spend up to 30 years in diapause.
- A major distinction between the existing types of dormancy is whether they are direct and immediate responses to environmental cues or are programmed and anticipatory. Torpor in warm-blooded animals and quiescence in insects can occur at any stage of development in an immediate response to an environmental challenge like cold, heat, or food scarcity. But other types of dormancy, like hibernation in mammals and diapause in insects, are predictive and involve an elaborate preparatory stage initiated long before a seasonal change occurs.
- Réaumur is better known for his octogesimal temperature scale from 1730, which sets the freezing point of water at 0 and the boiling point at 80.
- Translated from old French, as quoted here (with some omissions for brevity).
- Wheeler made significant contributions to insect embryology and pioneered the study of social insects, particularly ants. He was also the academic grandfather of another renowned researcher of insect eusociality, E. O. Wilson.
- The timing on this varies across species, as some species do not switch to anaerobic metabolism until several days after diapause has begun.
- Extensive experiments on chemical coaxers and disruptors of diapause come from the lab of David Denlinger, a foremost researcher of insect diapause at Ohio State University. Many other labs around the world also contributed to this body of work.
- Crop rotation typically means alternating the so-called cash and cover crops, though, at it simplest, only cash crops can be rotated. Cash crops (like corn or wheat) are those that yield a product to be sold on the market, while cover crops (like nitrogen-rich clover or other legumes) improve soil quality but lack market value. Since better soil quality also improves cash crop yield, both crop types are recommended, though cover crops are not a strict necessity for successful farming. More elaborate crop rotation schemes involve a sequence of several carefully selected crops instead of just two.
- 10–16 percent before harvest, and a similar amount following harvest (as storage pests). Postharvest losses to corn and wheat amount to as much as $2.5 billion annually.
- As reported by one affected farmer in the 1880s, “the corn does not have roots sufficient to support it, anything like a fresh breeze blowing it down, there being scarcely any brace roots.”
- Francis Marion Webster, the author of the first historical review of corn rootworm biology and its spread in the U.S., notes that in the wake of the Civil War, as the price of pork increased, growing corn as hog feed became very profitable. So farmers started replacing wheat fields with corn, and it is possible that such abundance of corn (perhaps through the spreading pollen) attracted corn rootworms that previously were only rarely seen in the existing fields. In a matter of a few years in the 1870s-80s, they multiplied to a degree that was no longer manageable though crop rotation.
- For one, the anti-herbivore defensive molecules like proteinase inhibitors produced by soybean plants inhibit the digestion of the plant tissues by the western corn rootworm.
- Interestingly, western corn rootworms, unlike their northern cousins, developed an ability to lay eggs on soybeans and feed on them, absent from original populations that could only lay eggs in cornfields. This is their way to bypass crop rotation.
- Insect parasitism was first documented in China in 1096, by Lu Dian (1042–1102) who observed and described the full life cycle of a tachinid parasitoid that pesters silkworms. But in Europe, parasitoids weren’t described until the second half of the 17th century, through the works of Johannes Goedaert (1617–1668), Francesco Redi (1626–1698) — who both misunderstood the parasitoid life cycle — and Jan Jacob Swammerdam (1637–1680) who first accurately describe it. Other notable contributions to understanding parasitoid life cycles were by the German-Dutch artist Maria Sybilla Merian (1647–1717) and Antoni van Leeuwenhoek (1632–1723), the father of microscopy.
- Parasitoids can also parasitize each other, resulting in hyperparasitism.
- Another example is Trichogramma brassicae, a parasitoid used as a biological control agent against the European corn borer. T. brassicae diapauses at a prepupal stage after exposure to cold temperatures. Keeping diapausing T. brassicaeprepupae at 3 ℃ allows up to nine months of storage for commercial production of these parasitoids.
- Other commercially reared silkmoth species include Antheraea mylitta that makes Tasar/Tussah silk, Chinese tussah moth Antheraea pernyi and Antheraea assamensis that makes golden-yellow muga silk.
- Most widely used bumble bee species are Bombus terrestris from Eurasia and Bombus impatiens from North America.
- In artificial long days and high rearing temperature (30-35℃).
- In cricket and mealworm farming, it is also a temperature-responsive quiescence, rather than true diapause, that is used for long-term storage and transportation. Commercially used cricket and mealworm species (Acheta domesticus, Gryllus bimaculatus and Tenebrio molitor) don’t naturally undergo diapause.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.