Atomic-Scale Protein Filters

Cells are crowded and frenzied places. But their interiors still contain more order than their surroundings.
Inside a cell, molecular machines convert raw inputs into highly ordered structures; DNA coils into chromosomes and proteins fold into precise three-dimensional shapes. Outside the cell, however, ions drift without gradients, while DNA and proteins exist mostly as scattered building blocks. Only a thin membrane separates the managed chaos inside from the high entropy outside.
If a cell’s membrane is punctured, the fluid within — made mostly of water — drains away, and the cell swiftly dies. And yet, the membrane must let molecules in (food, nutrients, ions) and out (waste, toxins, messages) for the cell to survive. The membrane does so by means of hyperspecific proteins that allow only particular molecules to pass. It is thanks to these atomic-scale filters that life can exist at all.
{{signup}}
What makes some of these filters remarkable is that they work without any “added” energy. Separating water from protons, or potassium from sodium, seems like it ought to require a great deal of resources. And yet, the only energy a cell requires to do so is that used for building the proteins themselves. In other words, evolution has figured out how to perform (nearly) energy-free filtration at a scale that keeps cells alive in an environment that would otherwise overwhelm them.
Two types of protein filters, aquaporin and the potassium ion channel, offer particularly elegant examples. Aquaporins let billions of water molecules pass in and out each second in a single file line, while blocking protons. Potassium channels conduct about 100 million K⁺ ions per second yet decisively reject Na⁺, a slightly smaller ion with the same charge. Both rely on geometry alone (namely, the precise placement of amino acids) to achieve their selectivity. And both demonstrate how seemingly complex problems are often solved in biology simply by positioning the correct atom, with the appropriate charge, at the perfect place.
Aquaporins were first theorized to exist in 1970 by two physiologists at the University of California, Berkeley. These scientists noticed that, when human red blood cells were treated with chemicals that latch onto cysteine, an amino acid found in many membrane-embedded proteins, the blood cells’ permeability dropped by about tenfold. Their finding came at a time when scientists believed that water passively moved into or out of cells through the cell membrane itself. They were the first to suggest, instead, that proteins were, by some as yet unknown mechanism, involved with the movement of water.
The protein responsible was not discovered until 1992 by a team of scientists led by Peter Agre at Johns Hopkins University. While purifying Rh antigens1 from red blood cells, an additional, unknown protein kept turning up. Agre’s team called it CHIP28.2
To figure out its function, the researchers injected frog eggs with RNA molecules encoding the CHIP28 protein. Doing so caused the eggs to swell up and burst, hinting that the mystery protein was some kind of water channel. Follow-up studies, from inhibitor assays in frog eggs to reconstitution in artificial membranes, later confirmed that CHIP28 was a dedicated water channel. In 2000, a high-resolution structure of the protein, renamed aquaporin, was published in Nature.
“The atomic model provides a possible molecular explanation to a longstanding puzzle in physiology,” the authors wrote. Namely, “how membranes can be freely permeable to water but impermeable to protons.”
Early structural imaging revealed that each aquaporin protein is made from six alpha-helices, or looping rods that run back and forth through the cell membrane. These helices form an hourglass shape, such that the channel is cone-shaped at both ends and narrow in the middle. The two ends of aquaporin mirror each other, with their point of symmetry occurring at the narrowest part of the hourglass.3
As water molecules flow from outside to inside the cell, the first obstacle they encounter is the ar/R filter. This is a cluster of four bulky or positively-charged amino acids, including arginine, situated inside the aquaporin channel. The positively charged amino acid forces away sodium ions and loose protons, while the bulky amino acids help to narrow the protein’s center to the width, roughly, of a single water molecule.
Once a water molecule moves past this first filter, it quickly encounters a second, called the NPA motif (short for asparagine–proline–alanine). There are two NPA filters pointing at each other inside the aquaporin.

When a water molecule passes the ar/R filter and moves deeper into the channel, it latches onto the asparagine amino acid in the first NPA motif. But then — almost instantaneously — the local electric field at the channel’s midpoint forces the water molecule to rotate 180° and latch onto the asparagine in the second NPA motif. In other words, these duelling NPA filters force each water molecule to flip around inside the aquaporin, thus breaking them away from any hitchhiking ions. Incredibly, this happens billions of times each second.
There are at least 13 different types of aquaporins in human cells, and nine different aquaporins are found in kidney cells alone. Some aquaporins transport not only water, but also small molecules like glycerol. It is even thought that some aquaporins may move gases — such as CO2, NH3, NO, O2 — across the membrane.
A second kind of atomic-scale filter is the potassium ion channel. These channels, too, span the cell membrane. But instead of transporting water, they admit potassium (K+) ions. Potassium channels are especially important in the brain. After a neuron triggers an action potential, it is these filters that open up and allow potassium to rush into the cell, thus restoring the neuron to its resting potential in preparation for its next firing.
The first clues about potassium channels came, yet again, not from biochemistry but from physiology. In the 1940s and early 1950s, Alan Hodgkin and Andrew Huxley used the squid giant axon as a model system to study how electrical signals travel along nerves. The squid giant axon stretches several centimeters in length, making it large enough to handle the direct insertion of electrodes. With this setup, and their later invention of the voltage-clamp, Hodgkin and Huxley could hold the membrane at a fixed voltage and watch the underlying currents run across the axon.
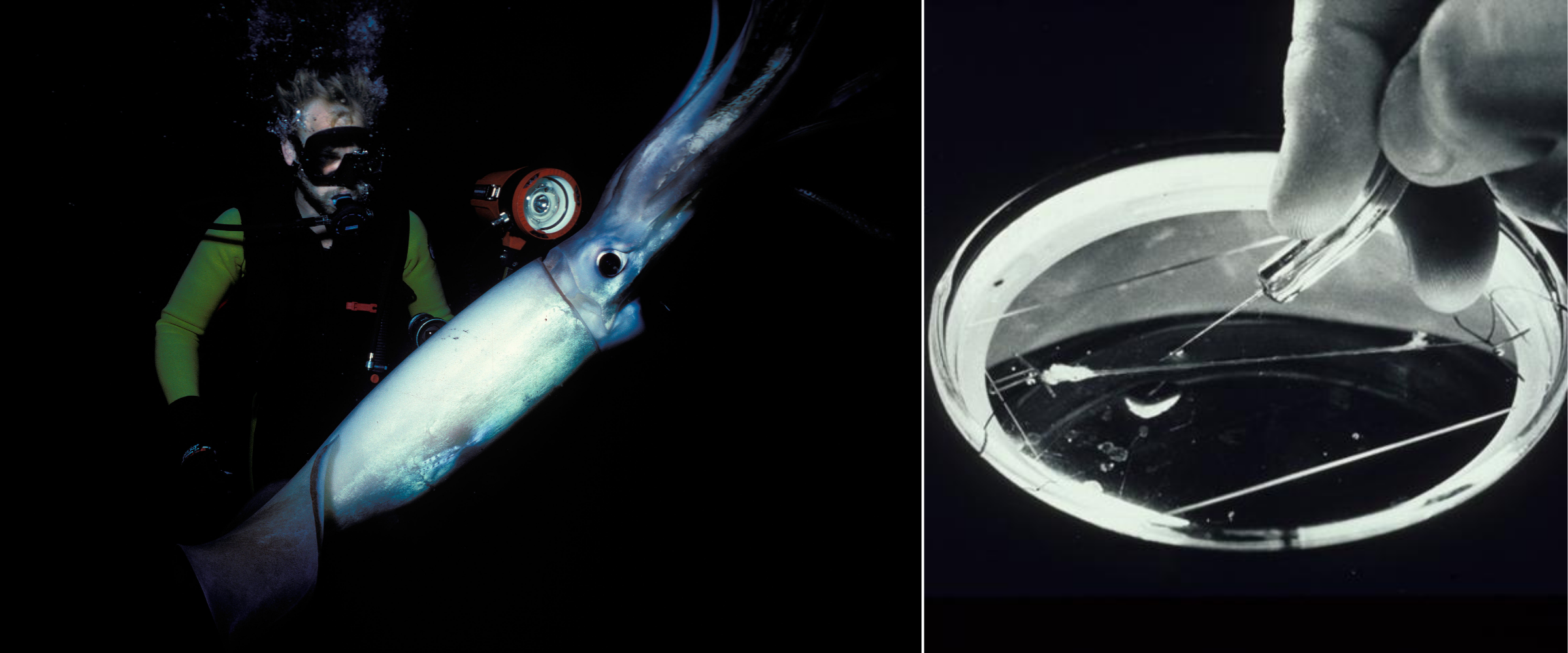
What they found, over the course of many years of research, was that action potentials in the axon were caused by a fast, transient inward current (sodium ions), followed by a slower outward current (potassium ions). Hodgkin and Huxley’s discovery of the action potential, and its mechanism, earned them a Nobel Prize in 1963.
The two men never figured out the proteins responsible for those potentials, however. That breakthrough came only in the 1980s, when researchers studying fruit flies with a mutant “Shaker” phenotype — flies whose muscles shook under ether anesthesia — traced the defect to a single gene. Cloning and sequencing the Shaker gene revealed that it encoded a voltage-gated potassium channel, thus proving Hodgkin and Huxley’s predictions at the molecular level. The protein structure was finally solved by Roderick MacKinnon’s team at Rockefeller University in 1998.
A single potassium channel funnels in 100 million ions per second while being 10,000-times more permeable to potassium than sodium. This selectivity is remarkable given that these two ions both carry a positive charge and are of similar size.4
The mechanism lies in the potassium channel’s interior, which is lined with oxygen atoms. Normally, potassium floats surrounded by a shell of water molecules, and stripping away those water molecules is energetically costly. But the potassium channel positions its oxygens (via a protein motif called P-loops) so that K+ ions shed their water and grab onto the protein instead. Na+, being slightly smaller, can’t make contact with all the oxygen molecules encircling the channel at once and so gets filtered out by geometry alone.
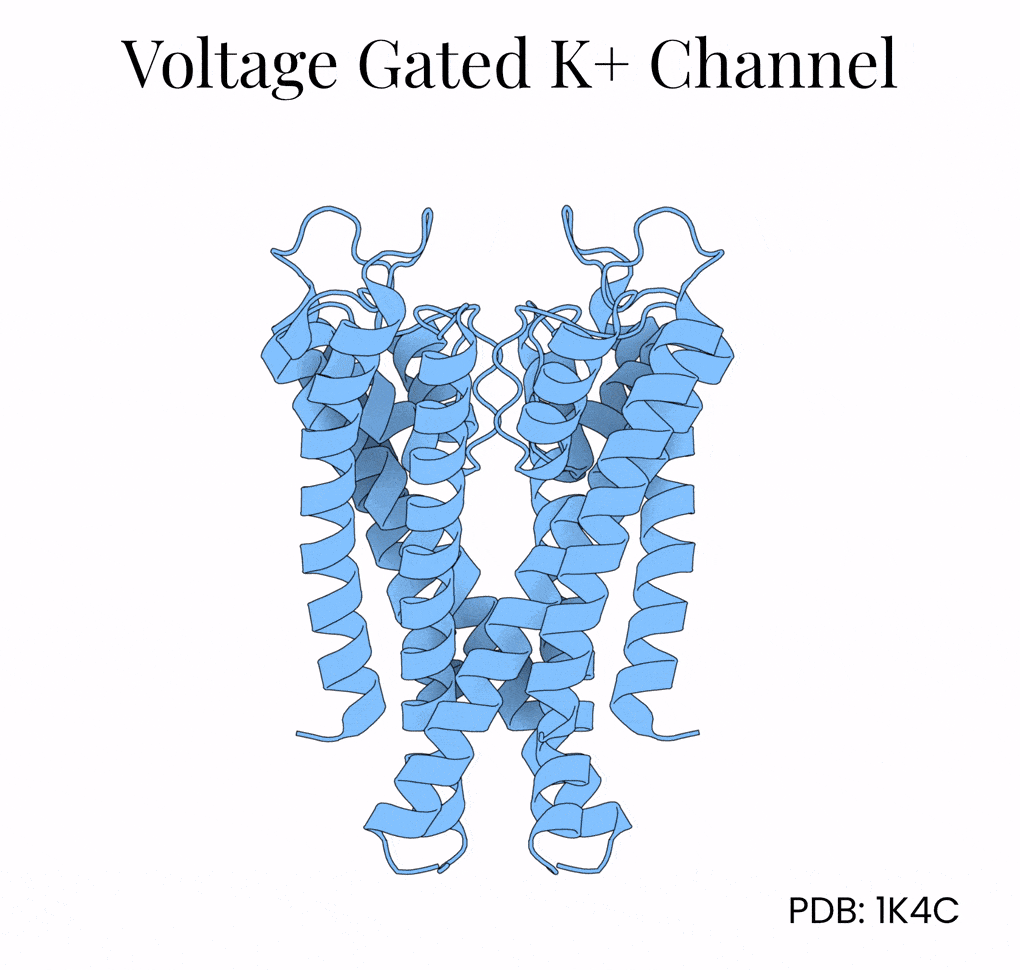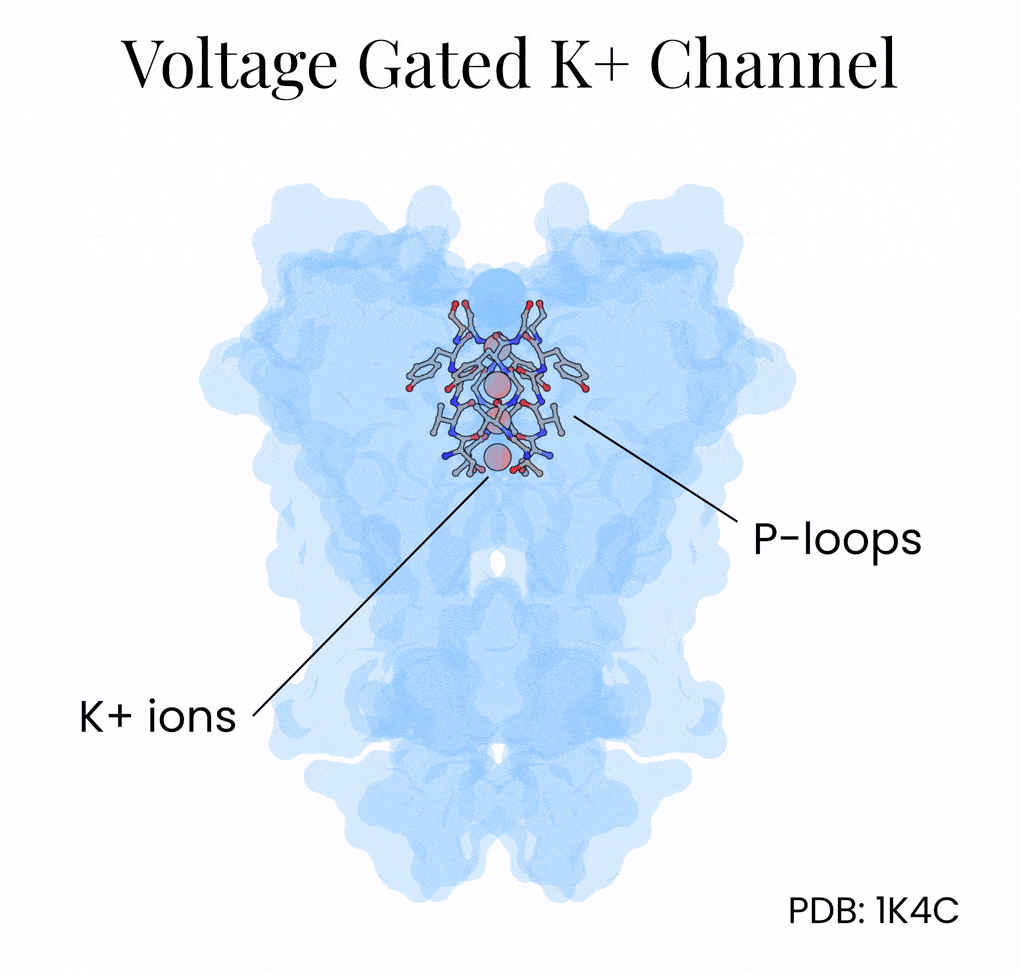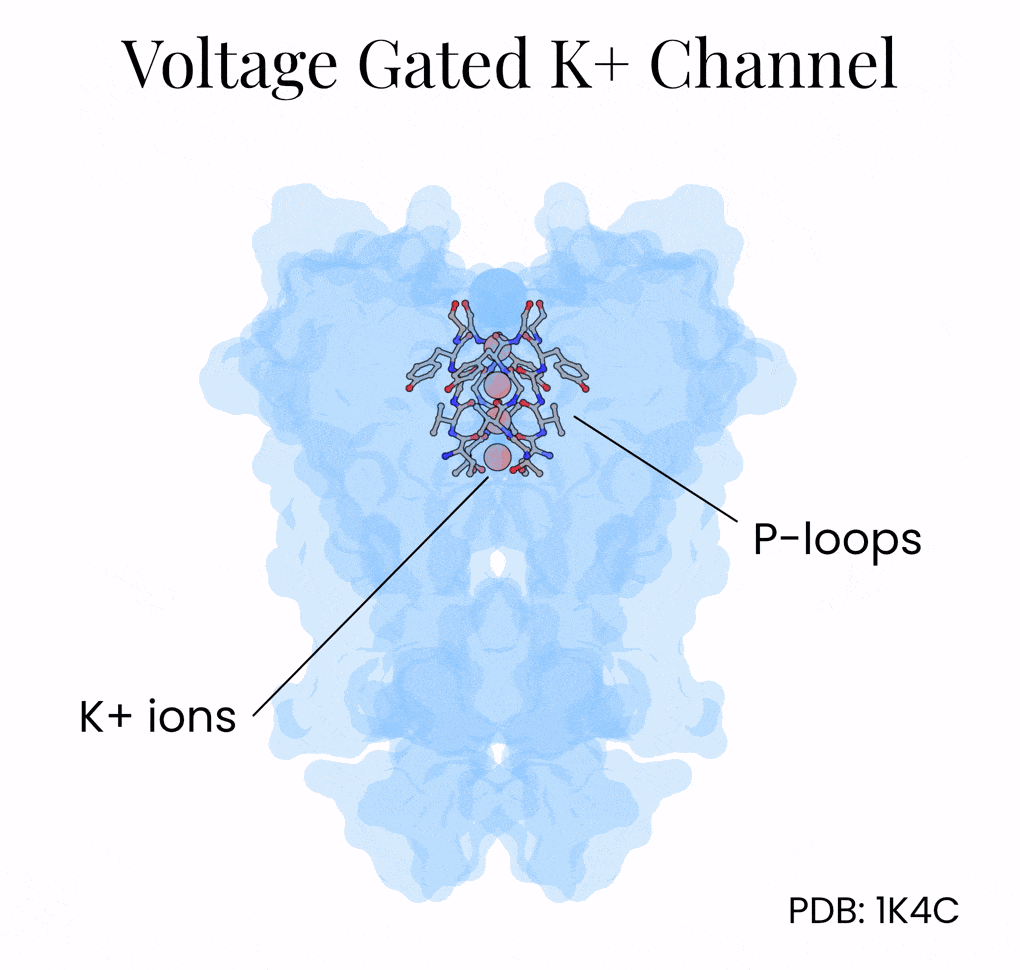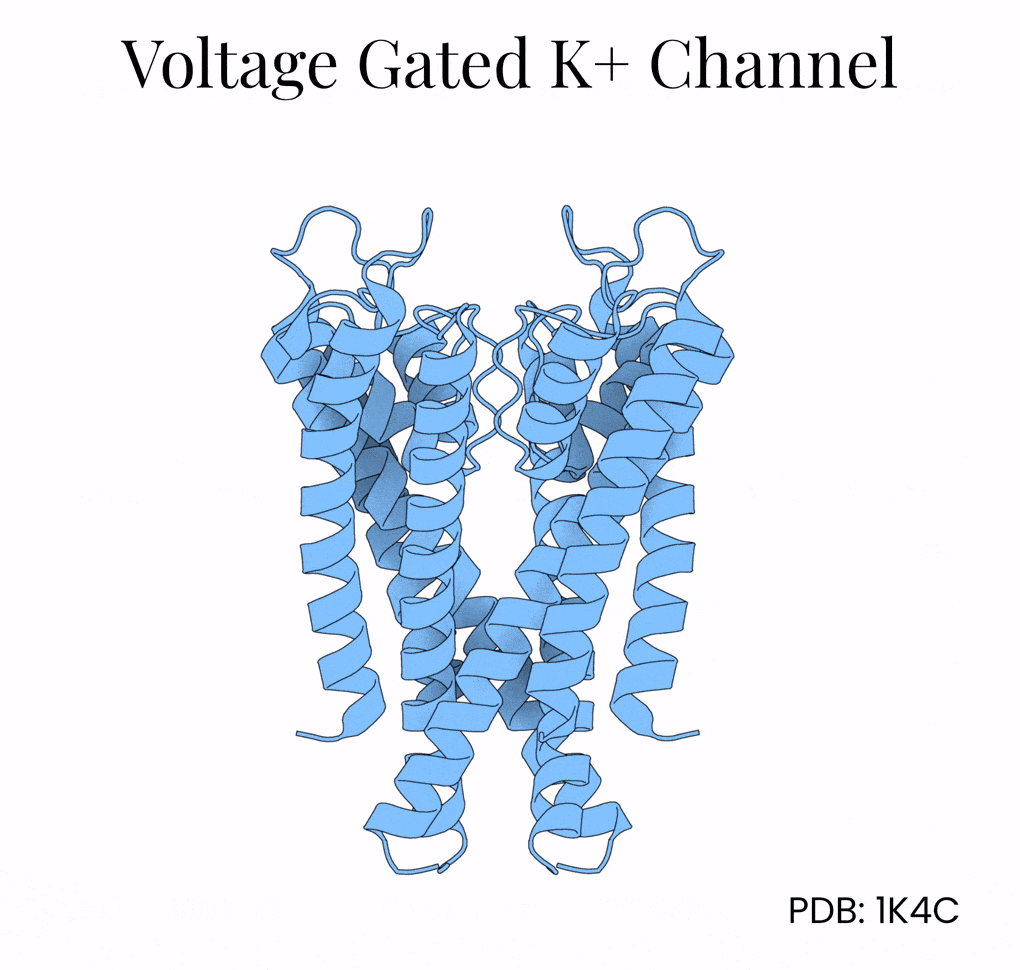
This geometry explains the potassium channel’s selectivity, but what explains its speed?
Once again, the answer came from structural biology. Researchers crystallized the potassium channel and then soaked the crystals in rubidium and cesium; two ions that behave similarly to potassium but scatter X-rays more strongly. These resolved structures showed that the inside of the potassium channel, which is 12 Å long, holds as many as four potassium ions at the same time, separated by about 3 Å of space.5 This distance is just close enough for the ions — held in space by oxygens within the channel — to repel one another.
The channel harnesses this repulsive force as an atomic propulsion system. When a new potassium ion enters from above, it nudges the others forward and pushes out the ion at the bottom. This “knock-on” mechanism helps explain how the channel can be both extremely selective and also incredibly fast.
What makes these filters so compelling is that they show how small details add up to enormous precision. In aquaporins and potassium channels, a single amino acid placed just so decides whether molecules pass or not. (This idea feels reminiscent of a J.S. Bach quote regarding the piano: “There’s nothing remarkable about it. All one has to do is hit the right keys at the right time and the instrument plays itself.”) Simply put, selectivity in biology rests on geometry and charge, arranged at the scale of angstroms.6
These filters also show that often, in biology, solutions to complicated problems (like how to separate out individual ions when many others carry the same charge) are often strikingly simple. Cells are bags of vibrating molecules, and they only work if the right molecules reach the appropriate places at the correct times. Much of cell biology lies downstream from this basic idea.
{{divider}}
Niko McCarty is a founding editor of Asimov Press.
Illustrations by Ella Watkins-Dulaney.
Cite: McCarty, Niko. “Atomic-Scale Protein Filters.” Asimov Press (2025). https://doi.org/10.62211/82gy-21uq
Footnotes
- A surface protein that determines the “+” or “-” part of a person’s blood type.
- The “28” refers to its size, which was 28 kilodaltons or about 250 amino acids in total.
- It’s thought that modern aquaporins evolved through a gene duplication event. This would explain their perfect symmetry.
- A potassium ion has a Pauling radius (a measure of how far its electron cloud extends from the nucleus) of 1.33 Angstroms, compared to 0.95 for the sodium ion.
- An angstrom (Å) is equivalent to one ten-billionth, or 10⁻¹⁰, of a meter. It is named after a Swedish physicist, Anders Jonas Ångström.
- Two of the people who revealed these elegant filtration mechanisms, Agre and MacKinnon, shared the 2003 Nobel Prize in Chemistry “for discoveries concerning channels in cell membranes.”
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.
Always free. No ads. Richly storied.